Cell potency

The potency of a cell specifies its differentiation potential, or potential to differentiate into different cell types.[1]
Totipotency
Totipotency is the ability of a single cell to divide and produce all the differentiated cells in an organism, including extraembryonic tissues.[2] Totipotent cells include spores and zygotes. [3] In some organisms, cells can dedifferentiate and regain totipotency. For example, a plant cutting or callus can be used to grow an entire plant.
Human development begins when a sperm fertilizes an egg and creates a single totipotent cell (zygote). In the first hours after fertilization, this cell divides into identical totipotent cells, which can later develop into any of the three germ layers of a human (endoderm, mesoderm, or ectoderm) and into cells of the cytotrophoblast layer or syncytiotrophoblast layer of the placenta. After reaching the 16-cell stage, the totipotent cells of the morula differentiate into cells that will eventually become either the blastocyst's Inner cell mass or the outer trophoblasts. Approximately four days after fertilization and after several cycles of cell division, these totipotent cells begin to specialize. The inner cell mass, the source of embryonic stem cells, is pluripotent, not totipotent.
Research on Caenorhabditis elegans suggests that multiple mechanisms including RNA regulation may play a role in maintaining totipotency at different stages of development in some species.[4]
Pluripotency
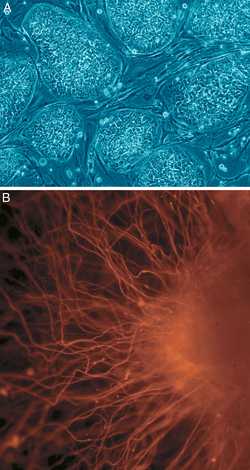
A: Cell colonies that are not yet differentiated.
B: Nerve cell
In cell biology, pluripotency (from the Latin plurimus, meaning very many, and potens, meaning having power) refers to a stem cell that has the potential to differentiate into any of the three germ layers: endoderm (interior stomach lining, gastrointestinal tract, the lungs), mesoderm (muscle, bone, blood, urogenital), or ectoderm (epidermal tissues and nervous system).[5] Pluripotent stem cells can give rise to any fetal or adult cell type. However, alone they cannot develop into a fetal or adult organism because they lack the potential to contribute to extraembryonic tissue, such as the placenta.
Induced pluripotency
Induced pluripotent stem cells,[6][7] commonly abbreviated as iPS cells are a type of pluripotent stem cell artificially derived from a non-pluripotent cell, typically an adult somatic cell, by inducing a "forced" expression of certain genes.
Multipotency
Multipotent progenitor cells have the potential to give rise to cells from multiple, but a limited number of lineages. An example of a multipotent stem cell is a hematopoietic cell — a blood stem cell that can develop into several types of blood cells, but cannot develop into brain cells or other types of cells. At the end of the long series of cell divisions that form the embryo are cells that are terminally differentiated, or that are considered to be permanently committed to a specific function. Another example is the mesenchymal stem cell, which can differentiate into osteoblasts, chondrocytes, and adipocytes[8] .
Scientists [who?] have long held the opinion that differentiated cells cannot be altered or caused to behave in any way other than the way in which they have been naturally committed. New research, however, has called that assumption into question.[9] In recent stem cell experiments, scientists have been able to persuade blood stem cells to behave like neurons, or brain cells- a process known as transdifferentiation. Scientists now believe that stem cell research could reveal far more vital information about our bodies than was previously known. There is also continuing research to see if it is possible to make multipotent cells into pluripotent cells.
The induced pluripotency of somatic cells into undifferentiated iPS cells was originally hailed as the end of the controversial use of embryonic stem cells. However, iPS cells are highly tumorigenic, and, despite advances,[6] were never approved for clinical stage research in the United States. It was recently determined that the somatic expression of combined transcription factors can directly induce other defined somatic cell fates; researchers identified three neural-lineage-specific transcription factors that could directly convert mouse fibroblasts (skin cells) into fully functional neurons.[9] This result challenges the terminal nature of cellular differentiation and the integrity of lineage commitment; and implies that with the proper tools, all cells are totipotent and may form all kinds of tissue.
An extremely rich source for mesenchymal stem cells is the developing tooth bud of the mandibular third molar. The stem cells eventually form enamel (ectoderm), dentin, dental pulp, blood vessels, and nervous tissues, including a minimum of 29 different unique end organs [citation needed]. Because of extreme ease in collection at 8–10 years of age before calcification and minimal to no morbidity will probably constitute a major source for personal banking, research and multiple therapies. These stem cells have been shown capable of producing hepatocytes [citation needed].
Oligopotency
In biology, oligopotency is the ability of progenitor cells to differentiate into a few cell types. It is a degree of potency. Examples of oligopotent stem cells are the lymphoid or myeloid stem cells.[1] Examples of progenitor cells are Vascular Stem Cells that have the capacity to become both Endothelial or smooth muscle cells.
Unipotency
In cell biology, a unipotent cell is one that has the capacity to differentiate into only one cell type. Hepatocytes, which constitute most of the liver, are unipotent. The liver's ability to regenerate from as little as 25% of its original mass is attributed to this property.[10] A close synonym for unipotent cell is precursor cell.
Notes
- ^ a b Hans R. Schöler (2007). "The Potential of Stem Cells: An Inventory". In Nikolaus Knoepffler, Dagmar Schipanski, and Stefan Lorenz Sorgner (ed.). Human biotechnology as Social Challenge. Ashgate Publishing, Ltd. p. 28. ISBN 978-0-7546-5755-2.
{{cite book}}: CS1 maint: multiple names: editors list (link) Cite error: The named reference "Schoeler" was defined multiple times with different content (see the help page). - ^ "Regenerative Medicine Glossary". Regenerative Medicine. 4 (4s): S30. July 2009. doi:10.2217/rme.09.s1. PMID 19604041.
{{cite journal}}: More than one of|at=and|page=specified (help) - ^ Mitalipov S, Wolf D (2009). "Totipotency, pluripotency and nuclear reprogramming". Adv Biochem Eng Biotechnol. 114: 185–99. doi:10.1007/10_2008_45. PMC 2752493. PMID 19343304.
- ^ February 2006 report in Science
- ^ Byrne, James (2011-06-29). "The definition and etymology of the word pluripotency". eJournal of Cellular Biotechnology, 1;eP2.
- ^ a b Baker, Monya (2007-12-06). "Adult cells reprogrammed to pluripotency, without tumors". Nature Reports Stem Cells. doi:10.1038/stemcells.2007.124.
- ^ Kolata, Gina (2007-11-21). "Scientists Bypass Need for Embryo to Get Stem Cells". The New York Times. ISSN 0362-4331. Retrieved 2007-12-11.
- ^ Uccelli (2008). "Mesenchymal stem cells in health and disease". Nature Reviews. 8. doi:10.1038/nri2395.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b Vierbuchen T, Wernig M; et al. (2010). "Direct conversion of fibroblasts to functional neurons by defined factors". Nature. 463 (7284): 1035–41. doi:10.1038/nature08797. PMC 2829121. PMID 20107439.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - ^ Michael, Dr. Sandra Rose (2007). "Bio-Scalar Technology: Regeneration and Optimization of the Body-Mind Homeostasis" (PDF). 15th Annual AAAAM Conference: 2. Retrieved October 24, 2008.
