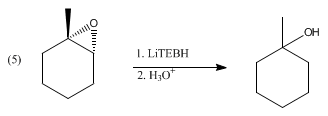Lithium triethylborohydride

| |
| Names | |
|---|---|
| IUPAC name
Lithium triethylborohydride
| |
| Other names
Superhydride
LiTEBH | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.040.963 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Li(C2H5)3BH | |
| Molar mass | 105.95 g/mol |
| Appearance | Colorless to yellow liquid |
| Density | 0.890 g/cm3, liquid |
| Boiling point | 66 °C for THF |
| reactive | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
highly flammable corrosive Causes burns Probable Carcinogen |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lithium triethylborohydride (LiEt3BH), commonly abbreviated to LiETBH or Superhydride, is a powerful and selective reducing agent used in inorganic and organic chemistry. LiTEBH is far more powerful than lithium borohydride and more powerful than lithium aluminium hydride (LAH) in many cases. One of the main advantages of LiTEBH is that it is safer than LAH.
LiTEBH rapidly reduces:
- Aldehydes, ketones, acid chlorides and esters to alcohols
- Lactones to diols
- Acid anhydrides to alcohols
- α,β-enones by 1,4-addition to give lithium enolates
- Disulfides to thiols
- Tertiary amides to an alcohol
History
Compounds of lithium hydride and sodium hydride with trialkylboranes such as LiTEBH were first discovered in the course of War Research in the period of 1942-45 at the University of Chicago by Professor H.C. Brown and Professor H.I. Schlesinger.[1]
Preparation
LiTEBH is most commonly produced by the reaction between lithium hydride (LiH) and triethylborane (Et3B) in tetrahydrofuran (THF)
- LiH + Et3B → LiEt3BH
which gives a very high yield of approximately 99%. The subsequent solution is collected by filtration of any excess LiH to give a crystal clear solution. LiTEBH in THF appears to be stable indefinitely under an inert atmosphere.
Structure and chemical properties
The structure of LiTEBH causes the compound to be a very strong hydride source. Hydrogen is more electronegative than boron which causes the B-H bond to be strongly polarized with boron having a partial positive charge and hydrogen having a partial negative charge. The ethyl groups on the boron also aids to this abnormal polarizability by removing additional electron density from the boron making it even more electropositive. This polarization of the B-Et and B-H bonds causes the hydrogen to be in the (-I) oxidation state instead of its usual (+I) oxidation state which leads to its high reactivity with atoms that can accept electrons to allow the hydrogen to go to its (+I) oxidation state.
Uses
Aldehydes and ketones rapidly utilizes 1 equivalent of LiTEBH to form the alcohol shown respectively in (1) and (2).
LiTEBH can be used to form the alcohol even when these compounds possess sterically hindered substituents as shown with 2,2,4,4-tetramethyl-3-pentanone in (3).
Esters and lactones rapidly take up 2 equivalents of LiTEBH, undergoing reduction to the alcohol and diol groups respectively. The example shown below involves the reduction of γ-butyrolactone to 1,4-butanediol which formed in a 94% yield in (4).[2]
The opening of the epoxide ring with LiTEBH proceeds with exceptional regio- and stereo- selectivity, yielding only the Markovnikov alcohol.[2] The example shown below is of 1,2-epoxybutane being reduced to give 1-methylcyclohexanol in (5).
The acetal and ketal will not be reduced by LiTEBH. LiTEBH can be used in the reductive cleavage of mesylates and tosylates.[3] LiTEBH can selectively deprotect tertiary N-acyl groups without affecting secondary amide functionality.[4] LiTEBH has also shown high reactivity toward ester groups by the selective reduction of the ester group of aromatic carboxylic acids to alcohols as shown in (6) and (7).[2]
LiTEBH can also effectively reduce pyridine and isoquinolines to piperidines and tetrahydroisoquinolines respectively.[5]
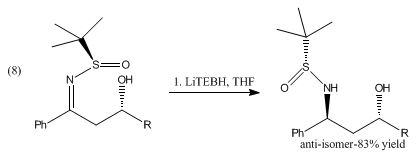
The reduction of β-hydroxysulfinyl imines with catecholborane and LiTEBH produces anti-1,3-amino alcohols shown in (8).[6]
Precautions
LiTEBH reacts violently and exothermically with water, alcohols, or acids releasing flammable hydrogen gas which can ignite explosively and the pyrophoric triethylborane vapor can ignite spontaneously. It can cause severe eye, skin, and respiratory tract burns.
References
- ^ Krishnamurthy, S. Aldrichim. Acta. 1974, 7, 55.
- ^ Brown, H.C.; Kim, S.C.; Krishnamurthy, S. J. Org. Chem. 1980, 45, 1-12.
- ^ Baer, H.H.; Mekarska-Falicki, M. Can. J. Chem. 1985, 63, 3043.
- ^ Tanaka, H.; Ogasawara, K. Tetrahedron Lett. 2002, 43, 4417.
- ^ Blough, B.E.; Carroll, F.I. Tetrahedron Lett. 1993, 34, 7239.
- ^ Kochi, T; Tang, T.P.; Ellman, J.A. J. Am. Chem. Soc. 2002, 124, 6518.