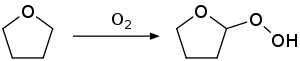Tetrahydrofuran
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxolane[1] | |||
| Systematic IUPAC name
1,4-Epoxybutane 1-Oxacyclopentane | |||
| Other names
Tetrahydrofuran
THF 1,4-Butylene oxide Cyclotetramethylene oxide fraction Furanidin Tetra-methylene oxide, Oxolane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | THF | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.389 | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H8O | |||
| Molar mass | 72.107 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Ether-like[2] | ||
| Density | 0.8876 g/cm3 at 20 °C, liquid [3] | ||
| Melting point | −108.4 °C (−163.1 °F; 164.8 K) | ||
| Boiling point | 66 °C (151 °F; 339 K)[4][3] | ||
| Miscible | |||
| Vapor pressure | 132 mmHg at 20 °C[2] | ||
Refractive index (nD)
|
1.4073 at 20 °C[3] | ||
| Viscosity | 0.48 cP at 25 °C | ||
| Structure | |||
| Envelope | |||
| 1.63 D (gas) | |||
| Hazards | |||
| GHS labelling: | |||
   [5] [5]
| |||
| Danger | |||
| H225, H302, H319, H335, H351[5] | |||
| P210, P280, P301+P312+P330, P305+P351+P338, P370+P378, P403+P235[5] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −14 °C (7 °F; 259 K) | ||
| Explosive limits | 2–11.8%[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
LC50 (median concentration)
|
21000 ppm (rat, 3 h)[6] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 200 ppm (590 mg/m3)[2] | ||
REL (Recommended)
|
TWA 200 ppm (590 mg/m3) ST 250 ppm (735 mg/m3)[2] | ||
IDLH (Immediate danger)
|
2000 ppm[2] | ||
| Related compounds | |||
Related heterocycles
|
Furan Pyrrolidine Dioxane | ||
Related compounds
|
Diethyl ether | ||
| Supplementary data page | |||
| Tetrahydrofuran (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers.[8] Being polar and having a wide liquid range, THF is a versatile solvent. It is an isomer of another solvent, butanone.
Production
[edit]About 200,000 tonnes of tetrahydrofuran are produced annually.[9] The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-butanediol. Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from condensation of acetylene with formaldehyde followed by hydrogenation.[8] DuPont developed a process for producing THF by oxidizing n-butane to crude maleic anhydride, followed by catalytic hydrogenation.[10] A third major industrial route entails hydroformylation of allyl alcohol followed by hydrogenation to 1,4-butanediol.
Other methods
[edit]THF can also be synthesized by catalytic hydrogenation of furan.[11][12] This allows certain sugars to be converted to THF via acid-catalyzed digestion to furfural and decarbonylation to furan,[13] although this method is not widely practiced. THF is thus derivable from renewable resources.
Applications
[edit]Polymerization
[edit]In the presence of strong acids, THF converts to a linear polymer called poly(tetramethylene ether) glycol (PTMEG), also known as polytetramethylene oxide (PTMO):
This polymer is primarily used to make elastomeric polyurethane fibers like Spandex.[14]
As a solvent
[edit]The other main application of THF is as an industrial solvent for polyvinyl chloride (PVC) and in varnishes.[8] It is an aprotic solvent with a dielectric constant of 7.6. It is a moderately polar solvent and can dissolve a wide range of nonpolar and polar chemical compounds.[15] THF is water-miscible and can form solid clathrate hydrate structures with water at low temperatures.[16]
THF has been explored as a miscible co-solvent in aqueous solution to aid in the liquefaction and delignification of plant lignocellulosic biomass for production of renewable platform chemicals and sugars as potential precursors to biofuels.[17] Aqueous THF augments the hydrolysis of glycans from biomass and dissolves the majority of biomass lignin making it a suitable solvent for biomass pretreatment.
THF is often used in polymer science. For example, it can be used to dissolve polymers prior to determining their molecular mass using gel permeation chromatography. THF dissolves PVC as well, and thus it is the main ingredient in PVC adhesives. It can be used to liquefy old PVC cement and is often used industrially to degrease metal parts.
THF is used as a component in mobile phases for reversed-phase liquid chromatography. It has a greater elution strength than methanol or acetonitrile, but is less commonly used than these solvents.
THF is used as a solvent in 3D printing when printing with PLA, PETG and substantially similar filaments. It can be used to clean clogged 3D printer parts, to remove extruder lines and add a shine to the finished product as well as to solvent weld printed parts.
Laboratory use
[edit]In the laboratory, THF is a popular solvent when its water miscibility is not an issue. It is more basic than diethyl ether[18] and forms stronger complexes with Li+, Mg2+, and boranes. It is a popular solvent for hydroboration reactions and for organometallic compounds such as organolithium and Grignard reagents.[19] Thus, while diethyl ether remains the solvent of choice for some reactions (e.g., Grignard reactions), THF fills that role in many others, where strong coordination is desirable and the precise properties of ethereal solvents such as these (alone and in mixtures and at various temperatures) allows fine-tuning modern chemical reactions.
Commercial THF contains substantial water that must be removed for sensitive operations, e.g. those involving organometallic compounds. Although THF is traditionally dried by distillation from an aggressive desiccant such as elemental sodium, molecular sieves have been shown to be superior water scavengers.[20]
Reaction with hydrogen sulfide
[edit]In the presence of a solid acid catalyst, THF reacts with hydrogen sulfide to give tetrahydrothiophene.[21]
Lewis basicity
[edit]
THF is a Lewis base that bonds to a variety of Lewis acids such as I2, phenols, triethylaluminum and bis(hexafluoroacetylacetonato)copper(II). THF has been classified in the ECW model and it has been shown that there is no one order of base strengths.[23] Many complexes are of the stoichiometry MCl3(THF)3.[24]
Precautions
[edit]THF is a relatively acutely nontoxic solvent, with the median lethal dose (LD50) comparable to that for acetone. However, chronic exposure is suspected of causing cancer.[5][25] Reflecting its remarkable solvent properties, it penetrates the skin, causing rapid dehydration. THF readily dissolves latex and thus should be handled with nitrile rubber gloves. It is highly flammable.
One danger posed by THF is its tendency to form the explosive compound 2-hydroperoxytetrahydrofuran upon reaction with air:
To minimize this problem, commercial supplies of THF are often stabilized with butylated hydroxytoluene (BHT). Distillation of THF to dryness is unsafe because the explosive peroxides can concentrate in the residue.
Related compounds
[edit]Tetrahydrofurans
[edit]

The tetrahydrofuran ring is found in diverse natural products including lignans, acetogenins, and polyketide natural products.[26] Diverse methodology has been developed for the synthesis of substituted THFs.[27]
Oxolanes
[edit]Tetrahydrofuran is one of the class of pentic cyclic ethers called oxolanes. There are seven possible structures, namely,[28]
- Monoxolane, the root of the group, synonymous with tetrahydrofuran
- 1,3-dioxolane
- 1,2-dioxolane
- 1,2,4-trioxolane
- 1,2,3-trioxolane
- tetroxolane
- pentoxolane
See also
[edit]- Polytetrahydrofuran
- 2-Methyltetrahydrofuran
- Trapp mixture
- Other cyclic ethers: oxirane (C
2H
4O), oxetane (C
3H
6O), oxane (C
5H
10O)
References
[edit]- ^ "New IUPAC Organic Nomenclature - Chemical Information BULLETIN" (PDF).
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0602". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c Baird, Zachariah Steven; Uusi-Kyyny, Petri; Pokki, Juha-Pekka; Pedegert, Emilie; Alopaeus, Ville (6 Nov 2019). "Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-compounds". International Journal of Thermophysics. 40 (11): 102. Bibcode:2019IJT....40..102B. doi:10.1007/s10765-019-2570-9.
- ^ NIST Chemistry WebBook. http://webbook.nist.gov
- ^ a b c d Record of Tetrahydrofuran in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2 June 2020.
- ^ a b "Tetrahydrofuran". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "New Environment Inc. - NFPA Chemicals". Newenv.com. Retrieved 2016-07-16.
- ^ a b c Müller, Herbert. "Tetrahydrofuran". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_221. ISBN 978-3527306732.
- ^ Karas, Lawrence; Piel, W. J. (2004). "Ethers". Kirk‑Othmer Encyclopedia of Chemical Technology. John Wiley & Sons.
- ^ Budavari, Susan, ed. (2001), The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (13th ed.), Merck, ISBN 0911910131
- ^ Morrison, Robert Thornton; Boyd, Robert Neilson (1972). Organic Chemistry (2nd ed.). Allyn and Bacon. p. 569.
- ^ Starr, Donald; Hixon, R. M. (1943). "Tetrahydrofuran". Organic Syntheses; Collected Volumes, vol. 2, p. 566.
- ^ Hoydonckx, H. E.; Rhijn, W. M. Van; Rhijn, W. Van; Vos, D. E. De; Jacobs, P. A. (2007), "Furfural and Derivatives", Ullmann's Encyclopedia of Industrial Chemistry, American Cancer Society, doi:10.1002/14356007.a12_119.pub2, ISBN 978-3-527-30673-2
- ^ Pruckmayr, Gerfried; Dreyfuss, P.; Dreyfuss, M. P. (1996). "Polyethers, Tetrahydrofuran and Oxetane Polymers". Kirk‑Othmer Encyclopedia of Chemical Technology. John Wiley & Sons.
- ^ "Chemical Reactivity". Michigan State University. Archived from the original on 2010-03-16. Retrieved 2010-02-15.
- ^ "NMR–MRI study of clathrate hydrate mechanisms" (PDF). Fileave.com. Archived from the original (PDF) on 2011-07-11. Retrieved 2010-02-15.
- ^ Cai, Charles; Zhang, Taiying; Kumar, Rajeev; Wyman, Charles (13 August 2013). "THF co-solvent enhances hydrocarbon fuel precursor yields from lignocellulosic biomass". Green Chemistry. 15 (11): 3140–3145. doi:10.1039/C3GC41214H.
- ^ Lucht, B. L.; Collum, D. B. (1999). "Lithium Hexamethyldisilazide: A View of Lithium Ion Solvation through a Glass-Bottom Boat". Accounts of Chemical Research. 32 (12): 1035–1042. doi:10.1021/ar960300e.
- ^ Elschenbroich, C.; Salzer, A. (1992). Organometallics: A Concise Introduction (2nd ed.). Weinheim: Wiley-VCH. ISBN 3-527-28165-7.
- ^ Williams, D. B. G.; Lawton, M. (2010). "Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants". Journal of Organic Chemistry. 75 (24): 8351–4. doi:10.1021/jo101589h. PMID 20945830. S2CID 17801540.
- ^ Swanston, Jonathan. "Thiophene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_793.pub2. ISBN 978-3527306732.
- ^ F.A. Cotton; S.A. Duraj; G.L. Powell; W.J. Roth (1986). "Comparative Structural Studies of the First Row Early Transition Metal(III) Chloride Tetrahydrofuran Solvates". Inorg. Chim. Acta. 113: 81. doi:10.1016/S0020-1693(00)86863-2.
- ^ Vogel G. C.; Drago, R. S. (1996). "The ECW Model". Journal of Chemical Education. 73 (8): 701–707. Bibcode:1996JChEd..73..701V. doi:10.1021/ed073p701.
- ^ Manzer, L. E. "Tetrahydrofuran Complexes of Selected Early Transition Metals," Inorganic Synthesis. 21, 135–140, (1982).
- ^ "Material Safety Data Sheet Tetrahydrofuran, 99.5+%, for spectroscopy". Fisher Scientific. Retrieved 2022-07-27.
- ^ Lorente, Adriana; Lamariano-Merketegi, Janire; Albericio, Fernando; Álvarez, Mercedes (2013). "Tetrahydrofuran-Containing Macrolides: A Fascinating Gift from the Deep Sea". Chemical Reviews. 113 (7): 4567–4610. doi:10.1021/cr3004778. PMID 23506053.
- ^ Wolfe, John P.; Hay, Michael B. (2007). "Recent advances in the stereoselective synthesis of tetrahydrofurans". Tetrahedron. 63 (2): 261–290. doi:10.1016/j.tet.2006.08.105. PMC 1826827. PMID 18180807.
- ^ Cremer, Dieter (1983). "Theoretical Determination of Molecular Structure and Conformation. XI. The Puckering of Oxolanes". Israel Journal of Chemistry. 23: 72–84. doi:10.1002/ijch.198300010.
General reference
[edit]- Loudon, G. Mark (2002). Organic Chemistry (4th ed.). New York: Oxford University Press. p. 318. ISBN 978-0-9815194-3-2.
External links
[edit]- International Chemical Safety Card 0578
- NIOSH Pocket Guide to Chemical Hazards
- U.S. OSHA info on THF Archived 2017-05-02 at the Wayback Machine
- "2-Methyltetrahydrofuran, An alternative to Tetrahydrofuran and Dichloromethane". Sigma-Aldrich. Retrieved 2007-05-23.




![{\displaystyle n\,{\ce {C4H8O}}\quad {\xrightarrow[{{\text{strong}} \atop {\text{acid}}}]{}}\quad {\bigl [}\!\!\!{\ce {-CH2CH2CH2CH2O -}}\!\!\!{\bigr ]}_{n}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1ec263360fef59fda69155ee527d1528b2944f4f)