Mesoporous silica
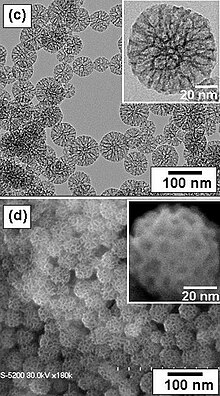
Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (<2 nm) and macroporous (>50 nm). Mesoporous silica is a relatively recent development in nanotechnology. The most common types of mesoporous nanoparticles are MCM-41 and SBA-15.[2] Research continues on the particles, which have applications in catalysis, drug delivery and imaging.[3] Mesoporous ordered silica films have been also obtained with different pore topologies.[4]
A compound producing mesoporous silica was patented around 1970.[5][6][7] It went almost unnoticed[8] and was reproduced in 1997.[9] Mesoporous silica nanoparticles (MSNs) were independently synthesized in 1990 by researchers in Japan.[10] They were later produced also at Mobil Corporation laboratories[11] and named Mobil Composition of Matter (or Mobil Crystalline Materials, MCM).[12]
Six years later, silica nanoparticles with much larger (4.6 to 30 nanometer) pores were produced at the University of California, Santa Barbara.[13] The material was named Santa Barbara Amorphous type material, or SBA-15. These particles also have a hexagonal array of pores.
The researchers who invented these types of particles planned to use them as molecular sieves. Today, mesoporous silica nanoparticles have many applications in medicine, biosensors,[14] thermal energy storage,[15] water/gas filtration [16] and imaging.
Synthesis
[edit]

Mesoporous silica nanoparticles are synthesized by reacting tetraethyl orthosilicate with a template made of micellar rods. The result is a collection of nano-sized spheres or rods that are filled with a regular arrangement of pores. The template can then be removed by washing with a solvent adjusted to the proper pH.[3]
Mesoporous particles can also be synthesized using a simple sol-gel method[1] such as the Stöber process, or a spray drying method.[17] Tetraethyl orthosilicate is also used with an additional polymer monomer (as a template).
However, TEOS is not the most effective precursor for synthesizing such particles; a better precursor is (3-Mercaptopropyl)trimethoxysilane, often abbreviated to MPTMS. Use of this precursor drastically reduces the chance of aggregation and ensures more uniform spheres.[18]
Drug delivery
[edit]The large surface area of the pores allows the particles to be filled with a drug or a cytotoxin. Like a Trojan Horse, the particles will be taken up by certain biological cells through endocytosis, depending on what chemicals are attached to the outside of the spheres. Some types of cancer cells will take up more of the particles than healthy cells will, giving researchers hope that MCM-41 will one day be used to treat certain types of cancer.[3][19][20]
Ordered mesoporous silica (e.g. SBA-15,[21] TUD-1,[22] HMM-33,[1] and FSM-16[23]) also show potential to boost the in vitro and in vivo dissolution of poorly water-soluble drugs. Many drug-candidates coming from drug discovery suffer from a poor water solubility. An insufficient dissolution of these hydrophobic drugs in the gastrointestinal fluids strongly limits the oral bioavailability. One example is itraconazole which is an antimycoticum known for its poor aqueous solubility. Upon introduction of itraconazole-on-SBA-15 formulation in simulated gastrointestinal fluids, a supersaturated solution is obtained giving rise to enhanced transepithelial intestinal transport.[24] Also the efficient uptake into the systemic circulation of SBA-15 formulated itraconazole has been demonstrated in vivo (rabbits and dogs).[25] This approach based on SBA-15 yields stable formulations[26] and can be used for a wide variety of poorly water-soluble compounds.[27]
Biosensors
[edit]The structure of these particles allows them to be filled with a fluorescent dye that would normally be unable to pass through cell walls. The MSN material is then capped off with a molecule that is compatible with the target cells. When the MSNs are added to a cell culture, they carry the dye across the cell membrane. These particles are optically transparent, so the dye can be seen through the silica walls. The dye in the particles does not have the same problem with self-quenching that a dye in solution has. The types of molecules that are grafted to the outside of the MSNs will control what kinds of biomolecules are allowed inside the particles to interact with the dye.[28][29]
See also
[edit]References
[edit]- ^ a b c Nandiyanto, Asep Bayu Dani; Kim, Soon-Gil; Iskandar, Ferry; Okuyama, Kikuo (2009). "Synthesis of Silica Nanoparticles with Nanometer-Size Controllable Mesopores and Outer Diameters". Microporous and Mesoporous Materials. 120 (3): 447–453. doi:10.1016/j.micromeso.2008.12.019.
- ^ Katiyar, Amit; Yadav, Santosh; Smirniotis, Panagiotis G.; Pinto, Neville G. (July 2006). "Synthesis of ordered large pore SBA-15 spherical particles for adsorption of biomolecules". Journal of Chromatography A. 1122 (1–2): 13–20. doi:10.1016/j.chroma.2006.04.055. ISSN 0021-9673. PMID 16716334.
- ^ a b c Trewyn, Brian G; Nieweg, Jennifer A; Zhao, Yannan; Lin, Victor S.-Y. (2007). "Biocompatible mesoporous silica nanoparticles with different morphologies for animal cell membrane penetration". Chemical Engineering Journal. 137 (1): 23–29. doi:10.1016/j.cej.2007.09.045.
- ^ Innocenzi, Plinio (2022). Mesoporous ordered silica films. From self-assembly to order. Advances in Sol-Gel Derived Materials and Technologies. Springer. doi:10.1007/978-3-030-89536-5. ISBN 978-3-030-89535-8. S2CID 245147740.
- ^ Chiola, V.; Ritsko, J. E. and Vanderpool, C. D. "Process for producing low-bulk density silica." Application No. US 3556725D A filed on 26-Feb-1969; Publication No. US 3556725 A published on 19-Jan-1971
- ^ "Porous silica particles containing a crystallized phase and method" Application No. US 3493341D A filed on 23-Jan-1967; Publication No. US 3493341 A published on 03-Feb-1970
- ^ "Process for producing silica in the form of hollow spheres"; Application No. US 342525 A filed on 04-Feb-1964; Publication No. US 3383172 A published on 14-May-1968
- ^ Xu, Ruren; Pang, Wenqin; Yu, Jihong (2007). Chemistry of zeolites and related porous materials: synthesis and structure. Wiley-Interscience. p. 472. ISBN 978-0-470-82233-3.
- ^ Direnzo, F; Cambon, H; Dutartre, R (1997). "A 28-year-old synthesis of micelle-templated mesoporous silica". Microporous Materials. 10 (4–6): 283–286. doi:10.1016/S0927-6513(97)00028-X.
- ^ Yanagisawa, Tsuneo; Shimizu, Toshio; Kuroda, Kazuyuki; Kato, Chuzo (1990). "The preparation of alkyltrimethylammonium-kanemite complexes and their conversion to microporous materials". Bulletin of the Chemical Society of Japan. 63 (4): 988–992. doi:10.1246/bcsj.63.988.
- ^ Beck, J. S.; Vartuli, J. C.; Roth, W. J.; Leonowicz, M. E.; Kresge, C. T.; Schmitt, K. D.; Chu, C. T. W.; Olson, D. H.; Sheppard, E. W. (1992). "A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates". Journal of the American Chemical Society. 114 (27): 10834–10843. doi:10.1021/ja00053a020.
- ^ Trewyn, B. G.; Slowing, I. I.; Giri, S; Chen, H. T.; Lin, V. S. (2007). "Synthesis and Functionalization of a Mesoporous Silica Nanoparticle Based on the Sol–Gel Process and Applications in Controlled Release". Accounts of Chemical Research. 40 (9): 846–853. doi:10.1021/ar600032u. PMID 17645305.
- ^ Zhao, Dongyuan; Feng, Jianglin; Huo, Qisheng; Melosh, Nicholas; Fredrickson, Glenn H.; Chmelka, Bradley F.; Stucky, Galen D. (1998). "Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores". Science. 279 (5350): 548–52. Bibcode:1998Sci...279..548Z. doi:10.1126/science.279.5350.548. PMID 9438845.
- ^ Valenti G, Rampazzo R, Bonacchi S, Petrizza L, Marcaccio M, Montalti M, Prodi L, Paolucci F (2016). "Variable Doping Induces Mechanism Swapping in Electrogenerated Chemiluminescence of Ru(bpy)32+ Core−Shell Silica Nanoparticles". J. Am. Chem. Soc. 138 (49): 15935–15942. doi:10.1021/jacs.6b08239. hdl:11585/583548. PMID 27960352.
- ^ Mitran, Raul−Augustin; Berger, Daniela; Munteanu, Cornel; Matei, Cristian (2015). "Evaluation of Different Mesoporous Silica Supports for Energy Storage in Shape-Stabilized Phase Change Materials with Dual Thermal Responses". The Journal of Physical Chemistry C. 119 (27): 15177–15184. doi:10.1021/acs.jpcc.5b02608.
- ^ Ghajeri, Farnaz; Topalian, Zareh; Tasca, Andrea; Jafri, Syed Hassan Mujtaba; Leifer, Klaus; Norberg, Peter; Sjöström, Christer (2018-08-01). "Case study of a green nanoporous material from synthesis to commercialisation: Quartzene®". Current Opinion in Green and Sustainable Chemistry. 12: 101–109. doi:10.1016/j.cogsc.2018.07.003. ISSN 2452-2236. S2CID 139146490.
- ^ Nandiyanto, A. B. D.; Iskandar, F. & Okuyama, K. (2008). "Nano-sized Polymer Particle-Facilitated Preparation of Mesoporous Silica Particles Using a Spray Method". Chemistry Letters. 37 (10): 1040–1041. doi:10.1246/cl.2008.1040.
- ^ Sivanandini, M.; Dhami, Sukhdeep S.; Pabla, B.S.; Gupta, M.K. (January 2014). "Effect of 3-mercaptopropyltrimethoxysilane on Surface Finish and Material Removal Rate in Chemical Mechanical Polishing". Procedia Materials Science. 6: 528–537. doi:10.1016/j.mspro.2014.07.067.
- ^ Roggers, Robert; Kanvinde, Shrey; Boonsith, Suthida; Oupický, David (2014-10-01). "The Practicality of Mesoporous Silica Nanoparticles as Drug Delivery Devices and Progress Toward This Goal". AAPS PharmSciTech. 15 (5): 1163–1171. doi:10.1208/s12249-014-0142-7. ISSN 1530-9932. PMC 4179667. PMID 24871552.
- ^ Wani, Amit; Savithra, Galbokka H. Layan; Abyad, Ayat; Kanvinde, Shrey; Li, Jing; Brock, Stephanie; Oupický, David (2017-05-23). "Surface PEGylation of Mesoporous Silica Nanorods (MSNR): Effect on loading, release, and delivery of mitoxantrone in hypoxic cancer cells". Scientific Reports. 7 (1): 2274. doi:10.1038/s41598-017-02531-4. ISSN 2045-2322. PMC 5442097. PMID 28536462.
- ^ Mellaerts, Randy; Aerts, Caroline A.; Humbeeck, Jan Van; Augustijns, Patrick; Den Mooter, Guy Van; Martens, Johan A. (2007). "Enhanced release of itraconazole from ordered mesoporous SBA-15 silica materials". Chemical Communications (13): 1375–7. doi:10.1039/b616746b. PMID 17377687.
- ^ Heikkila, T; Salonen, J; Tuura, J; Hamdy, M; Mul, G; Kumar, N; Salmi, T; Murzin, D; et al. (2007). "Mesoporous silica material TUD-1 as a drug delivery system". International Journal of Pharmaceutics. 331 (1): 133–8. doi:10.1016/j.ijpharm.2006.09.019. PMID 17046183.
- ^ Tozuka, Yuichi; Wongmekiat, Arpansiree; Kimura, Kyoko; Moribe, Kunikazu; Yamamura, Shigeo; Yamamoto, Keiji (2005). "Effect of Pore Size of FSM-16 on the Entrapment of Flurbiprofen in Mesoporous Structures". Chemical & Pharmaceutical Bulletin. 53 (8): 974–977. doi:10.1248/cpb.53.974. PMID 16079530.
- ^ Mellaerts, Randy; Mols, Raf; Kayaert, Pieterjan; Annaert, Pieter; Van Humbeeck, Jan; Van Den Mooter, Guy; Martens, Johan A.; Augustijns, Patrick (2008). "Ordered mesoporous silica induces pH-independent supersaturation of the basic low solubility compound itraconazole resulting in enhanced transepithelial transport". International Journal of Pharmaceutics. 357 (1–2): 169–79. doi:10.1016/j.ijpharm.2008.01.049. PMID 18325700.
- ^ Mellaerts, Randy; Mols, Raf; Jammaer, Jasper A.G.; Aerts, Caroline A.; Annaert, Pieter; Van Humbeeck, Jan; Van Den Mooter, Guy; Augustijns, Patrick; Martens, Johan A. (2008). "Increasing the oral bioavailability of the poorly water soluble drug itraconazole with ordered mesoporous silica". European Journal of Pharmaceutics and Biopharmaceutics. 69 (1): 223–30. doi:10.1016/j.ejpb.2007.11.006. PMID 18164930.
- ^ Mellaerts, Randy; Houthoofd, Kristof; Elen, Ken; Chen, Hong; Van Speybroeck, Michiel; Van Humbeeck, Jan; Augustijns, Patrick; Mullens, Jules; Van Den Mooter, Guy; Martens, Johan A. (2010). "Aging behavior of pharmaceutical formulations of itraconazole on SBA-15 ordered mesoporous silica carrier material". Microporous and Mesoporous Materials. 130 (1–3): 154–161. doi:10.1016/j.micromeso.2009.10.026.
- ^ Van Speybroeck, Michiel; Barillaro, Valéry; Thi, Thao Do; Mellaerts, Randy; Martens, Johan; Van Humbeeck, Jan; Vermant, Jan; Annaert, Pieter; et al. (2009). "Ordered mesoporous silica material SBA-15: A broad-spectrum formulation platform for poorly soluble drugs". Journal of Pharmaceutical Sciences. 98 (8): 2648–58. doi:10.1002/jps.21638. PMID 19072861.
- ^ Trewyn, Brian G; Supratim, Giri; Slowing, Igor I; Lin, Victor S.-Y. (2007). "Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems". Chemical Communications (31): 3236–3245. doi:10.1039/b701744h. PMID 17668088.
- ^ Radu, Daniela R; Lai, Chen-Yu; Jeftinija, Ksenija; Rowe, Eric W; Jeftinija, Srdija & Lin, Victor S.-Y. (2004). "A Polyamidoamine Dendrimer-Capped Mesoporous Silica Nanosphere-Based Gene Transfection Reagent". Journal of the American Chemical Society. 126 (41): 13216–13217. doi:10.1021/ja046275m. PMID 15479063.
