Hydrogel
| Polymer science |
|---|
 |


A hydrogel is a biphasic material, a mixture of porous and permeable solids and at least 10% of water or other interstitial fluid.[1][2] The solid phase is a water insoluble three dimensional network of polymers, having absorbed a large amount of water or biological fluids.[2][3][4][5] Hydrogels have several applications, especially in the biomedical area, such as in hydrogel dressing. Many hydrogels are synthetic, but some are derived from natural materials.[6][7] The term "hydrogel" was coined in 1894.[8]

Chemistry
[edit]Classification
[edit]The crosslinks which bond the polymers of a hydrogel fall under two general categories: physical hydrogels and chemical hydrogels. Chemical hydrogels have covalent cross-linking bonds, whereas physical hydrogels have non-covalent bonds.[citation needed] Chemical hydrogels can result in strong reversible or irreversible gels due to the covalent bonding.[9] Chemical hydrogels that contain reversible covalent cross-linking bonds, such as hydrogels of thiomers being cross-linked via disulfide bonds, are non-toxic and are used in numerous medicinal products.[10][11][12] Physical hydrogels usually have high biocompatibility, are not toxic, and are also easily reversible by simply changing an external stimulus such as pH, ion concentration (alginate) or temperature (gelatine); they are also used for medical applications.[13][14][15][16][17] Physical crosslinks consist of hydrogen bonds, hydrophobic interactions, and chain entanglements (among others). A hydrogel generated through the use of physical crosslinks is sometimes called a 'reversible' hydrogel.[13] Chemical crosslinks consist of covalent bonds between polymer strands. Hydrogels generated in this manner are sometimes called 'permanent' hydrogels.
Hydrogels are prepared using a variety of polymeric materials, which can be divided broadly into two categories according to their origin: natural or synthetic polymers. Natural polymers for hydrogel preparation include hyaluronic acid, chitosan, heparin, alginate, gelatin and fibrin.[18] Common synthetic polymers include polyvinyl alcohol, polyethylene glycol, sodium polyacrylate, acrylate polymers and copolymers thereof.[6] Whereas natural hydrogels are usually non-toxic, and often provide other advantages for medical use, such as biocompatibility, biodegradability, antibiotic/antifungal effect and improve regeneration of nearby tissue, their stability and strength is usually much lower than synthetic hydrogels.[19] There are also synthetic hydrogels that can be used for medical applications, such as polyethylene glycol (PEG), polyacrylate, and polyvinylpyrrolidone (PVP).[20]
Preparation
[edit]
There are two suggested mechanisms behind physical hydrogel formation, the first one being the gelation of nanofibrous peptide assemblies, usually observed for oligopeptide precursors. The precursors self-assemble into fibers, tapes, tubes, or ribbons that entangle to form non-covalent cross-links. The second mechanism involves non-covalent interactions of cross-linked domains that are separated by water-soluble linkers, and this is usually observed in longer multi-domain structures.[21] Tuning of the supramolecular interactions to produce a self-supporting network that does not precipitate, and is also able to immobilize water which is vital for to gel formation. Most oligopeptide hydrogels have a β-sheet structure, and assemble to form fibers, although α-helical peptides have also been reported.[22][23] The typical mechanism of gelation involves the oligopeptide precursors self-assemble into fibers that become elongated, and entangle to form cross-linked gels.
One notable method of initiating a polymerization reaction involves the use of light as a stimulus. In this method, photoinitiators, compounds that cleave from the absorption of photons, are added to the precursor solution which will become the hydrogel. When the precursor solution is exposed to a concentrated source of light, usually ultraviolet irradiation, the photoinitiators will cleave and form free radicals, which will begin a polymerization reaction that forms crosslinks between polymer strands. This reaction will cease if the light source is removed, allowing the amount of crosslinks formed in the hydrogel to be controlled.[24] The properties of a hydrogel are highly dependent on the type and quantity of its crosslinks, making photopolymerization a popular choice for fine-tuning hydrogels. This technique has seen considerable use in cell and tissue engineering applications due to the ability to inject or mold a precursor solution loaded with cells into a wound site, then solidify it in situ.[25][24]
Physically crosslinked hydrogels can be prepared by different methods depending on the nature of the crosslink involved. Polyvinyl alcohol hydrogels are usually produced by the freeze-thaw technique. In this, the solution is frozen for a few hours, then thawed at room temperature, and the cycle is repeated until a strong and stable hydrogel is formed.[26] Alginate hydrogels are formed by ionic interactions between alginate and double-charged cations. A salt, usually calcium chloride, is dissolved into an aqueous sodium alginate solution, that causes the calcium ions to create ionic bonds between alginate chains.[27] Gelatin hydrogels are formed by temperature change. A water solution of gelatin forms an hydrogel at temperatures below 37–35 °C, as Van der Waals interactions between collagen fibers become stronger than thermal molecular vibrations.[28]
Peptide based hydrogels
[edit]Peptide based hydrogels possess exceptional biocompatibility and biodegradability qualities, giving rise to their wide use of applications, particularly in biomedicine;[2] as such, their physical properties can be fine-tuned in order to maximise their use.[2] Methods to do this are: modulation of the amino acid sequence, pH, chirality, and increasing the number of aromatic residues.[29] The order of amino acids within the sequence is crucial for gelation, as has been shown many times. In one example, a short peptide sequence Fmoc-Phe-Gly readily formed a hydrogel, whereas Fmoc-Gly-Phe failed to do so as a result of the two adjacent aromatic moieties being moved, hindering the aromatic interactions.[30][31] Altering the pH can also have similar effects, an example involved the use of the naphthalene (Nap) modified dipeptides Nap-Gly-Ala, and Nap- Ala-Gly, where a drop in pH induced gelation of the former, but led to crystallisation of the latter.[32] A controlled pH decrease method using glucono-δ-lactone (GdL), where the GdL is hydrolysed to gluconic acid in water is a recent strategy that has been developed as a way to form homogeneous and reproducible hydrogels.[33][34] The hydrolysis is slow, which allows for a uniform pH change, and thus resulting in reproducible homogenous gels. In addition to this, the desired pH can be achieved by altering the amount of GdL added. The use of GdL has been used various times for the hydrogelation of Fmoc and Nap-dipeptides.[33][34] In another direction, Morris et al reported the use of GdL as a 'molecular trigger' to predict and control the order of gelation.[35] Chirality also plays an essential role in gel formation, and even changing the chirality of a single amino acid from its natural L-amino acid to its unnatural D-amino acid can significantly impact the gelation properties, with the natural forms not forming gels.[36] Furthermore, aromatic interactions play a key role in hydrogel formation as a result of π- π stacking driving gelation, shown by many studies.[37][38]
Other
[edit]Hydrogels also possess a degree of flexibility very similar to natural tissue due to their significant water content. As responsive "smart materials", hydrogels can encapsulate chemical systems which upon stimulation by external factors such as a change of pH may cause specific compounds such as glucose to be liberated to the environment, in most cases by a gel–sol transition to the liquid state. Chemomechanical polymers are mostly also hydrogels, which upon stimulation change their volume and can serve as actuators or sensors.
-
-
A short-peptide-based hydrogel matrix, capable of holding about one hundred times its own weight in water. Developed as a medical dressing.
-
Photo of the same short-peptide-based hydrogel, held in forceps to demonstrate its stiffness and transparency.
Mechanical properties
[edit]Hydrogels have been investigated for diverse applications. By modifying the polymer concentration of a hydrogel (or conversely, the water concentration), the Young's modulus, shear modulus, and storage modulus can vary from 10 Pa to 3 MPa, a range of about five orders of magnitude.[40] A similar effect can be seen by altering the crosslinking concentration.[40] This much variability of the mechanical stiffness is why hydrogels are so appealing for biomedical applications, where it is vital for implants to match the mechanical properties of the surrounding tissues.[41] Characterizing the mechanical properties of hydrogels can be difficult especially due to the differences in mechanical behavior that hydrogels have in comparison to other traditional engineering materials. In addition to its rubber elasticity and viscoelasticity, hydrogels have an additional time dependent deformation mechanism which is dependent on fluid flow called poroelasticity. These properties are extremely important to consider while performing mechanical experiments. Some common mechanical testing experiments for hydrogels are tension, compression (confined or unconfined), indentation, shear rheometry or dynamic mechanical analysis.[40]
Hydrogels have two main regimes of mechanical properties: rubber elasticity and viscoelasticity:
Rubber elasticity
[edit]In the unswollen state, hydrogels can be modelled as highly crosslinked chemical gels, in which the system can be described as one continuous polymer network. In this case:
where G is the shear modulus, k is the Boltzmann constant, T is temperature, Np is the number of polymer chains per unit volume, ρ is the density, R is the ideal gas constant, and is the (number) average molecular weight between two adjacent cross-linking points. can be calculated from the swell ratio, Q, which is relatively easy to test and measure.[40]
For the swollen state, a perfect gel network can be modeled as:[40]
In a simple uniaxial extension or compression test, the true stress, , and engineering stress, , can be calculated as:
where is the stretch.[40]
Viscoelasticity
[edit]For hydrogels, their elasticity comes from the solid polymer matrix while the viscosity originates from the polymer network mobility and the water and other components that make up the aqueous phase.[42] Viscoelastic properties of a hydrogel is highly dependent on the nature of the applied mechanical motion. Thus, the time dependence of these applied forces is extremely important for evaluating the viscoelasticity of the material.[43]
Physical models for viscoelasticity attempt to capture the elastic and viscous material properties of a material. In an elastic material, the stress is proportional to the strain while in a viscous material, the stress is proportional to the strain rate. The Maxwell model is one developed mathematical model for linear viscoelastic response. In this model, viscoelasticity is modeled analogous to an electrical circuit with a Hookean spring, that represents the Young's modulus, and a Newtonian dashpot that represents the viscosity. A material that exhibit properties described in this model is a Maxwell material. Another physical model used is called the Kelvin-Voigt Model and a material that follow this model is called a Kelvin–Voigt material.[44] In order to describe the time-dependent creep and stress-relaxation behavior of hydrogel, a variety of physical lumped parameter models can be used.[40] These modeling methods vary greatly and are extremely complex, so the empirical Prony Series description is commonly used to describe the viscoelastic behavior in hydrogels.[40]
In order to measure the time-dependent viscoelastic behavior of polymers dynamic mechanical analysis is often performed. Typically, in these measurements the one side of the hydrogel is subjected to a sinusoidal load in shear mode while the applied stress is measured with a stress transducer and the change in sample length is measured with a strain transducer.[43] One notation used to model the sinusoidal response to the periodic stress or strain is:
in which G' is the real (elastic or storage) modulus, G" is the imaginary (viscous or loss) modulus.
Poroelasticity
[edit]Poroelasticity is a characteristic of materials related to the migration of solvent through a porous material and the concurrent deformation that occurs.[40] Poroelasticity in hydrated materials such as hydrogels occurs due to friction between the polymer and water as the water moves through the porous matrix upon compression. This causes a decrease in water pressure, which adds additional stress upon compression. Similar to viscoelasticity, this behavior is time dependent, thus poroelasticity is dependent on compression rate: a hydrogel shows softness upon slow compression, but fast compression makes the hydrogel stiffer. This phenomenon is due to the friction between the water and the porous matrix is proportional to the flow of water, which in turn is dependent on compression rate. Thus, a common way to measure poroelasticity is to do compression tests at varying compression rates.[45] Pore size is an important factor in influencing poroelasticity. The Kozeny–Carman equation has been used to predict pore size by relating the pressure drop to the difference in stress between two compression rates.[45]
Poroelasticity is described by several coupled equations, thus there are few mechanical tests that relate directly to the poroelastic behavior of the material, thus more complicated tests such as indentation testing, numerical or computational models are utilized. Numerical or computational methods attempt to simulate the three dimensional permeability of the hydrogel network.
Toughness and hysteresis
[edit]The toughness of a hydrogel refers to the ability of the hydrogel to withstand deformation or mechanical stress without fracturing or breaking apart. A hydrogel with high toughness can maintain its structural integrity and functionality under higher stress. Several factors contribute to the toughness of a hydrogel including composition, crosslink density, polymer chain structure, and hydration level. The toughness of a hydrogel is highly dependent on what polymer(s) and crosslinker(s) make up its matrix as certain polymers possess higher toughness and certain crosslinking covalent bonds are inherently stronger.[46] Additionally, higher crosslinking density generally leads to increased toughness by restricting polymer chain mobility and enhancing resistance to deformation. The structure of the polymer chains is also a factor in that, longer chain lengths and higher molecular weight leads to a greater number of entanglements and higher toughness.[47] A good balance (equilibrium) in the hydration of a hydrogel leads is important because too low hydration causes poor flexibility and toughness within the hydrogel, but too high of water content can cause excessive swelling, weakening the mechanical properties of the hydrogel.[48][49]

The hysteresis of a hydrogel refers to the phenomenon where there is a delay in the deformation and recovery of a hydrogel when it is subjected to mechanical stress and relieved of that stress. This occurs because the polymer chains within a hydrogel rearrange, and the water molecules are displaced, and energy is stored as it deforms in mechanical extension or compression.[50] When the mechanical stress is removed, the hydrogel begins to recover its original shape, but there may be a delay in the recovery process due to factors like viscoelasticity, internal friction, etc.[51] This leads to a difference between the stress-strain curve during loading and unloading. Hysteresis within a hydrogel is influenced by several factors including composition, crosslink density, polymer chain structure, and temperature.
The toughness and hysteresis of a hydrogel are especially important in the context of biomedical applications such as tissue engineering and drug delivery, as the hydrogel may need to withstand mechanical forces within the body, but also maintain mechanical performance and stability over time.[52] Most typical hydrogels, both natural and synthetic, have a positive correlation between toughness and hysteresis, meaning that the higher the toughness, the longer the hydrogel takes to recover its original shape and vice versa.[47] This is largely due to sacrificial bonds being the source of toughness within many of these hydrogels. Sacrificial bonds are non-covalent interactions such as hydrogen bonds, ionic interactions, and hydrophobic interactions, that can break and reform under mechanical stress.[53] The reforming of these bonds takes time, especially when there are more of them, which leads to an increase in hysteresis. However, there is currently research focused on the development of highly entangled hydrogels, which instead rely on the long chain length of the polymers and their entanglement to limit the deformation of the hydrogel, thereby increasing the toughness without increasing hysteresis as there is no need for the reformation of the bonds.[47]
Environmental response
[edit]The most commonly seen environmental sensitivity in hydrogels is a response to temperature.[54] Many polymers/hydrogels exhibit a temperature dependent phase transition, which can be classified as either an upper critical solution temperature (UCST) or lower critical solution temperature (LCST). UCST polymers increase in their water-solubility at higher temperatures, which lead to UCST hydrogels transitioning from a gel (solid) to a solution (liquid) as the temperature is increased (similar to the melting point behavior of pure materials). This phenomenon also causes UCST hydrogels to expand (increase their swell ratio) as temperature increases while they are below their UCST.[54] However, polymers with LCSTs display an inverse (or negative) temperature-dependence, where their water-solubility decreases at higher temperatures. LCST hydrogels transition from a liquid solution to a solid gel as the temperature is increased, and they also shrink (decrease their swell ratio) as the temperature increases while they are above their LCST.[54]
Applications can dictate for diverse thermal responses. For example, in the biomedical field, LCST hydrogels are being investigated as drug delivery systems due to being injectable (liquid) at room temp and then solidifying into a rigid gel upon exposure to the higher temperatures of the human body.[54] There are many other stimuli that hydrogels can be responsive to, including: pH, glucose, electrical signals, light, pressure, ions, antigens, and more.[54]
Additives
[edit]The mechanical properties of hydrogels can be fine-tuned in many ways beginning with attention to their hydrophobic properties.[54][55] Another method of modifying the strength or elasticity of hydrogels is to graft or surface coat them onto a stronger/stiffer support, or by making superporous hydrogel (SPH) composites, in which a cross-linkable matrix swelling additive is added.[7] Other additives, such as nanoparticles and microparticles, have been shown to significantly modify the stiffness and gelation temperature of certain hydrogels used in biomedical applications.[56][57][58]
Processing techniques
[edit]While a hydrogel's mechanical properties can be tuned and modified through crosslink concentration and additives, these properties can also be enhanced or optimized for various applications through specific processing techniques. These techniques include electro-spinning, 3D/4D printing, self-assembly, and freeze-casting. One unique processing technique is through the formation of multi-layered hydrogels to create a spatially-varying matrix composition and by extension, mechanical properties. This can be done by polymerizing the hydrogel matrixes in a layer by layer fashion via UV polymerization. This technique can be useful in creating hydrogels that mimic articular cartilage, enabling a material with three separate zones of distinct mechanical properties.[59]
Another emerging technique to optimize hydrogel mechanical properties is by taking advantage of the Hofmeister series. Due to this phenomenon, through the addition of salt solution, the polymer chains of a hydrogel aggregate and crystallize, which increases the toughness of the hydrogel. This method, called "salting out", has been applied to poly(vinyl alcohol) hydrogels by adding a sodium sulfate salt solution.[60] Some of these processing techniques can be used synergistically with each other to yield optimal mechanical properties. Directional freezing or freeze-casting is another method in which a directional temperature gradient is applied to the hydrogel is another way to form materials with anisotropic mechanical properties. Utilizing both the freeze-casting and salting-out processing techniques on poly(vinyl alcohol) hydrogels to induce hierarchical morphologies and anisotropic mechanical properties.[61] Directional freezing of the hydrogels helps to align and coalesce the polymer chains, creating anisotropic array honeycomb tube-like structures while salting out the hydrogel yielded out a nano-fibril network on the surface of these honeycomb tube-like structures. While maintaining a water content of over 70%, these hydrogels' toughness values are well above those of water-free polymers such as polydimethylsiloxane (PDMS), Kevlar, and synthetic rubber. The values also surpass the toughness of natural tendon and spider silk.[61]
Applications
[edit]Soft contact lenses
[edit]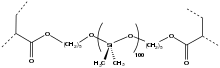
The dominant material for contact lenses are acrylate-siloxane hydrogels. They have replaced hard contact lenses. One of their most attractive properties is oxygen permeability, which is required since the cornea lacks vasculature.
Research
[edit]

- Scalephobicity and antifouling [63]
- Coatings for gas evolution reaction electrodes for efficient bubble detachment [64][65][66]
- Breast implants
- Contact lenses (silicone hydrogels, polyacrylamides, polymacon)
- Water sustainability: Hydrogels have emerged as promising materials platforms for solar-powered water purification,[67] water disinfection,[68] and atmospheric water generator.[69]
- Disposable diapers where they absorb urine, or in sanitary napkins[25]
- Dressings for healing of burn or other hard-to-heal wounds. Wound gels are excellent for helping to create or maintain a moist environment.
- EEG and ECG medical electrodes using hydrogels composed of cross-linked polymers (polyethylene oxide, polyAMPS and polyvinylpyrrolidone)
- Encapsulation of quantum dots
- Environmentally sensitive hydrogels (also known as 'smart gels' or 'intelligent gels'). These hydrogels have the ability to sense changes of pH, temperature, or the concentration of metabolite and release their load as result of such a change.[70][71][72]
- Fibers
- Glue
- Granules for holding soil moisture in arid areas
- Air bubble-repellent (superaerophobicity). Can improve the performance and stability of electrodes for water electrolysis.[73]
- Culturing cells: Hydrogel-coated wells have been used for cell culture.[74]
- Biosensors: Hydrogels that are responsive to specific molecules,[75] such as glucose or antigens, can be used as biosensors, as well as in DDS.[76]
- Cell carrier: Injectable hydrogels can be used to carry drugs or cells for applications in tissue regeneration or 3D bioprinting.[77][78][79] Hydrogels with reversible chemistry are required to allow for fluidization during injection/printing followed by self-healing of the original hydrogel structure.[80]
- Investigate cell biomechanical functions combined with holotomography microscopy
- Provide absorption, desloughing and debriding of necrotic and fibrotic tissue
- Tissue engineering scaffolds. When used as scaffolds, hydrogels may contain human cells to repair tissue. They mimic 3D microenvironment of cells.[81] Materials include agarose, methylcellulose, hyaluronan, elastin-like polypeptides, and other naturally derived polymers.
- Sustained-release drug delivery systems. Ionic strength, pH and temperature can be used as a triggering factor to control the release of the drug.[82]
- The swelling behavior exhibited by charged hydrogels can be used as a valuable tool for investigating interactions between charged polymers and various species, including multivalent ions, peptides, and proteins.[83] This response arises due to fluctuating osmotic swelling forces resulting from the exchange of counterions within the gel matrix. Particularly significant is its application in assessing the binding of peptide drugs to biopolymers within the body, as the swelling response of the gel can provide insights into these interactions.[84][85]
- Window coating/replacement: Hydrogels are under consideration for reducing infrared light absorption by 75%.[86] Another approach reduced interior temperature using a temperature-responsive hydrogel.[87]
- Thermodynamic electricity generation: When combined with ions allows for heat dissipation for electronic devices and batteries and converting the heat exchange to an electrical charge.[88]
- Water gel explosives
- Controlled release of agrochemicals (pesticides and fertilizer)
- Talin shock absorbing materials - protein-based hydrogels that can absorb supersonic impacts[89]
- Computational tasks, including emergent memory.[90]
Biomaterials
[edit]Implanted or injected hydrogels have the potential to support tissue regeneration by mechanical tissue support, localized drug or cell delivery,[2] local cell recruitement or immunomodulation, or encapsulation of nanoparticles for local photothermal therapy or brachytherapy.[80] Polymeric drug delivery systems have overcome challenges due to their biodegradability, biocompatibility, and anti-toxicity.[91][92] Materials such as collagen, chitosan, cellulose, and poly (lactic-co-glycolic acid) have been implemented extensively for drug delivery to organs such as eye,[93] nose, kidneys,[94] lungs,[95] intestines,[96] skin[97] and brain.[2] Future work is focused on reducing toxicity, improving biocompatibility, expanding assembly techniques[98]
Hydrogels have been considered as vehicles for drug delivery.[99][77][78][79] They can also be made to mimic animal mucosal tissues to be used for testing mucoadhesive properties.[100][101] They have been examined for use as reservoirs in topical drug delivery; particularly ionic drugs, delivered by iontophoresis.
References
[edit]![]() This article incorporates text by Jessica Hutchinson available under the CC BY 3.0 license.
This article incorporates text by Jessica Hutchinson available under the CC BY 3.0 license.
- ^ Wichterle, O.; Lím, D. (1960-01-01). "Hydrophilic Gels for Biological Use". Nature. 185 (4706): 117–118. Bibcode:1960Natur.185..117W. doi:10.1038/185117a0. ISSN 0028-0836. S2CID 4211987.
- ^ a b c d e f Ghosh, Shampa; Ghosh, Soumya; Sharma, Hitaishi; Bhaskar, Rakesh; Han, Sung Soo; Sinha, Jitendra Kumar (2024-01-01). "Harnessing the power of biological macromolecules in hydrogels for controlled drug release in the central nervous system: A review". International Journal of Biological Macromolecules. 254: 127708. doi:10.1016/j.ijbiomac.2023.127708. ISSN 0141-8130. S2CID 264944892.
- ^ Shrivastava, Priya; Vishwakarma, Nikhar; Gautam, Laxmikant; Vyas, Suresh P. (2023), "Magnetically responsive polymeric gels and elastomeric system(s) for drug delivery", Smart Polymeric Nano-Constructs in Drug Delivery, Elsevier, pp. 129–150, doi:10.1016/b978-0-323-91248-8.00012-x, ISBN 978-0-323-91248-8, retrieved 2023-01-16
- ^ Fundamental Biomaterials: Polymers. 2018. doi:10.1016/c2016-0-03544-1. ISBN 9780081021941.
- ^ Polymer Science: A Comprehensive Reference. Elsevier. 2012. doi:10.1016/c2009-1-28406-1. ISBN 978-0-08-087862-1.
- ^ a b Cai W, Gupta RB (2012). "Hydrogels". Kirk-Othmer Encyclopedia of Chemical Technology. pp. 1–20. doi:10.1002/0471238961.0825041807211620.a01.pub2. ISBN 978-0471238966.
- ^ a b Ahmed EM (March 2015). "Hydrogel: Preparation, characterization, and applications: A review". Journal of Advanced Research. 6 (2): 105–121. doi:10.1016/j.jare.2013.07.006. PMC 4348459. PMID 25750745.
- ^ Bemmelen JM (1907). "Der Hydrogel und das kristallinische Hydrat des Kupferoxydes". Zeitschrift für Chemie und Industrie der Kolloide. 1 (7): 213–214. doi:10.1007/BF01830147. S2CID 197928622.
- ^ Nikolić, Ljubiša B.; Zdravković, Aleksandar S.; Nikolić, Vesna D.; Ilić-Stojanović, Snežana S. (2018), Mondal, Md. Ibrahim H. (ed.), "Synthetic Hydrogels and Their Impact on Health and Environment", Cellulose-Based Superabsorbent Hydrogels, Cham: Springer International Publishing, pp. 1–29, doi:10.1007/978-3-319-76573-0_61-1, ISBN 978-3-319-76573-0, retrieved 2023-01-17
- ^ Summonte, S; Racaniello, GF; Lopedota, A; Denora, N; Bernkop-Schnürch, A (2021). "Thiolated polymeric hydrogels for biomedical application: Cross-linking mechanisms". Journal of Controlled Release. 330: 470–482. doi:10.1016/j.jconrel.2020.12.037. PMID 33359581. S2CID 229694027.
- ^ Federer, C; Kurpiers, M; Bernkop-Schnürch, A (2021). "Thiolated Chitosans: A Multi-talented Class of Polymers for Various Applications". Biomacromolecules. 22 (1): 24–56. doi:10.1021/acs.biomac.0c00663. PMC 7805012. PMID 32567846.
- ^ Leichner, C; Jelkmann, M; Bernkop-Schnürch, A (2019). "Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature". Advanced Drug Delivery Reviews. 151–152: 191–221. doi:10.1016/j.addr.2019.04.007. PMID 31028759. S2CID 135464452.
- ^ a b Rosales, Adrianne M.; Anseth, Kristi S. (2016-02-02). "The design of reversible hydrogels to capture extracellular matrix dynamics". Nature Reviews Materials. 1 (2): 15012. Bibcode:2016NatRM...115012R. doi:10.1038/natrevmats.2015.12. ISSN 2058-8437. PMC 5714327. PMID 29214058.
- ^ Jeong, Byeongmoon; Kim, Sung Wan; Bae, You Han (2002-01-17). "Thermosensitive sol-gel reversible hydrogels". Advanced Drug Delivery Reviews. 54 (1): 37–51. doi:10.1016/s0169-409x(01)00242-3. ISSN 0169-409X. PMID 11755705.
- ^ Yan, Yonggan; Xu, Shulei; Liu, Huanxi; Cui, Xin; Shao, Jinlong; Yao, Peng; Huang, Jun; Qiu, Xiaoyong; Huang, Chuanzhen (2020-05-20). "A multi-functional reversible hydrogel adhesive". Colloids and Surfaces A: Physicochemical and Engineering Aspects. 593: 124622. doi:10.1016/j.colsurfa.2020.124622. ISSN 0927-7757. S2CID 213116098.
- ^ Monteiro, O. A.; Airoldi, C. (November 1999). "Some studies of crosslinking chitosan-glutaraldehyde interaction in a homogeneous system". International Journal of Biological Macromolecules. 26 (2–3): 119–128. doi:10.1016/s0141-8130(99)00068-9. ISSN 0141-8130. PMID 10517518.
- ^ Zhang, Zhen; He, Chaoliang; Chen, Xuesi (2018-09-27). "Hydrogels based on pH-responsive reversible carbon–nitrogen double-bond linkages for biomedical applications". Materials Chemistry Frontiers. 2 (10): 1765–1778. doi:10.1039/C8QM00317C. ISSN 2052-1537.
- ^ Kharkar PM, Kiick KL, Kloxin AM (September 2013). "Designing degradable hydrogels for orthogonal control of cell microenvironments". Chemical Society Reviews. 42 (17): 7335–7372. doi:10.1039/C3CS60040H. PMC 3762890. PMID 23609001.
- ^ Jeong, Kwang-Hun; Park, Duckshin; Lee, Young-Chul (July 2017). "Polymer-based hydrogel scaffolds for skin tissue engineering applications: a mini-review". Journal of Polymer Research. 24 (7): 112. doi:10.1007/s10965-017-1278-4. ISSN 1022-9760. S2CID 136085690.
- ^ Gdansk University of Technology, Chemical Faculty, Polymer Technology Department, 80-233 Gdansk, ul Narutowicza 11/12; Gibas, Iwona; Janik, Helena (2010-12-15). "Review: Synthetic Polymer Hydrogels for Biomedical Applications". Chemistry & Chemical Technology. 4 (4): 297–304. doi:10.23939/chcht04.04.297.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Dooling LJ, Tirrell DA (2013). "Peptide and Protein Hydrogels.". Polymeric and self assembled hydrogels: from fundamental understanding to applications. Monographs in supramolecular chemistry. Vol. 11. Cambridge, UK: Royal Society of Chemistry. pp. 93–124. ISBN 978-1-84973-561-2.
- ^ Mehrban N, Zhu B, Tamagnini F, et al. (June 2015). "Functionalized α-Helical Peptide Hydrogels for Neural Tissue Engineering". ACS Biomaterials Science & Engineering. 1 (6): 431–439. doi:10.1021/acsbiomaterials.5b00051. PMC 4517957. PMID 26240838.
- ^ Banwell EF, Abelardo ES, Adams DJ, et al. (July 2009). "Rational design and application of responsive alpha-helical peptide hydrogels". Nature Materials. 8 (7): 596–600. Bibcode:2009NatMa...8..596B. doi:10.1038/nmat2479. PMC 2869032. PMID 19543314.
- ^ a b Choi JR, Yong KW, Choi JY, Cowie AC (January 2019). "Recent advances in photo-crosslinkable hydrogels for biomedical applications". BioTechniques. 66 (1): 40–53. doi:10.2144/btn-2018-0083. PMID 30730212.
- ^ a b Caló E, Khutoryanskiy VV (2015). "Biomedical applications of hydrogels: A review of patents and commercial products". European Polymer Journal. 65: 252–267. Bibcode:2015EurPJ..65..252C. doi:10.1016/j.eurpolymj.2014.11.024.
- ^ Adelnia, Hossein; Ensandoost, Reza; Shebbrin Moonshi, Shehzahdi; Gavgani, Jaber Nasrollah; Vasafi, Emad Izadi; Ta, Hang Thu (2022-02-05). "Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future". European Polymer Journal. 164: 110974. Bibcode:2022EurPJ.16410974A. doi:10.1016/j.eurpolymj.2021.110974. hdl:10072/417476. ISSN 0014-3057. S2CID 245576810.
- ^ Augst, Alexander D.; Kong, Hyun Joon; Mooney, David J. (2006-08-07). "Alginate Hydrogels as Biomaterials". Macromolecular Bioscience. 6 (8): 623–633. doi:10.1002/mabi.200600069. ISSN 1616-5187. PMID 16881042.
- ^ Jaipan, Panupong; Nguyen, Alexander; Narayan, Roger J. (2017-09-01). "Gelatin-based hydrogels for biomedical applications". MRS Communications. 7 (3): 416–426. Bibcode:2017MRSCo...7..416J. doi:10.1557/mrc.2017.92. ISSN 2159-6867.
- ^ Fichman G, Gazit E (April 2014). "Self-assembly of short peptides to form hydrogels: design of building blocks, physical properties and technological applications". Acta Biomaterialia. 10 (4): 1671–1682. doi:10.1016/j.actbio.2013.08.013. PMID 23958781.
- ^ Jayawarna V, Ali M, Jowitt TA, et al. (2006-03-03). "Nanostructured Hydrogels for Three-Dimensional Cell Culture Through Self-Assembly of Fluorenylmethoxycarbonyl–Dipeptides". Advanced Materials. 18 (5): 611–614. Bibcode:2006AdM....18..611J. doi:10.1002/adma.200501522. ISSN 0935-9648. S2CID 136880479.
- ^ Orbach R, Adler-Abramovich L, Zigerson S, et al. (September 2009). "Self-assembled Fmoc-peptides as a platform for the formation of nanostructures and hydrogels". Biomacromolecules. 10 (9): 2646–2651. doi:10.1021/bm900584m. PMID 19705843.
- ^ Adams DJ, Morris K, Chen L, et al. (2010). "The delicate balance between gelation and crystallisation: structural and computational investigations". Soft Matter. 6 (17): 4144. Bibcode:2010SMat....6.4144A. doi:10.1039/c0sm00409j. ISSN 1744-683X.
- ^ a b Chen L, Morris K, Laybourn A, et al. (April 2010). "Self-assembly mechanism for a naphthalene-dipeptide leading to hydrogelation". Langmuir. 26 (7): 5232–5242. doi:10.1021/la903694a. PMID 19921840.
- ^ a b Adams DJ, Mullen LM, Berta M, et al. (2010). "Relationship between molecular structure, gelation behaviour and gel properties of Fmoc-dipeptides". Soft Matter. 6 (9): 1971. Bibcode:2010SMat....6.1971A. doi:10.1039/b921863g. ISSN 1744-683X.
- ^ Morris KL, Chen L, Raeburn J, et al. (June 2013). "Chemically programmed self-sorting of gelator networks". Nature Communications. 4 (1): 1480. Bibcode:2013NatCo...4.1480M. doi:10.1038/ncomms2499. PMID 23403581.
- ^ Marchesan S, Waddington L, Easton CD, et al. (November 2012). "Unzipping the role of chirality in nanoscale self-assembly of tripeptide hydrogels". Nanoscale. 4 (21): 6752–6760. Bibcode:2012Nanos...4.6752M. doi:10.1039/c2nr32006a. hdl:11368/2841344. PMID 22955637.
- ^ Birchall LS, Roy S, Jayawarna V, et al. (2011). "Exploiting CH-π interactions in supramolecular hydrogels of aromatic carbohydrate amphiphiles". Chemical Science. 2 (7): 1349. doi:10.1039/c0sc00621a. ISSN 2041-6520.
- ^ Ma M, Kuang Y, Gao Y, et al. (March 2010). "Aromatic-aromatic interactions induce the self-assembly of pentapeptidic derivatives in water to form nanofibers and supramolecular hydrogels". Journal of the American Chemical Society. 132 (8): 2719–2728. doi:10.1021/ja9088764. PMID 20131781.
- ^ Kwon GH, Jeong GS, Park JY, et al. (September 2011). "A low-energy-consumption electroactive valveless hydrogel micropump for long-term biomedical applications". Lab on a Chip. 11 (17): 2910–2915. doi:10.1039/C1LC20288J. PMID 21761057.
- ^ a b c d e f g h i Oyen ML (January 2014). "Mechanical characterisation of hydrogel materials". International Materials Reviews. 59 (1): 44–59. Bibcode:2014IMRv...59...44O. doi:10.1179/1743280413Y.0000000022. ISSN 0950-6608. S2CID 136844625.
- ^ Los MJ, Hudecki A, Wiechec E (2018-11-07). Stem Cells and Biomaterials for Regenerative Medicine. Academic Press. ISBN 978-0-12-812278-5.
- ^ Tirella A, Mattei G, Ahluwalia A (October 2014). "Strain rate viscoelastic analysis of soft and highly hydrated biomaterials". Journal of Biomedical Materials Research. Part A. 102 (10): 3352–3360. doi:10.1002/jbm.a.34914. PMC 4304325. PMID 23946054.
- ^ a b Anseth KS, Bowman CN, Brannon-Peppas L (September 1996). "Mechanical properties of hydrogels and their experimental determination". Biomaterials. 17 (17): 1647–1657. doi:10.1016/0142-9612(96)87644-7. PMID 8866026.
- ^ Roylance D. ""Engineering viscoelasticity"" (PDF). Modules in Mechanics of Materials. Massachusetts Institute of Technology. Retrieved 11 May 2021.
- ^ a b Isobe N, Kimura S, Wada M, Deguchi S (November 2018). "Poroelasticity of cellulose hydrogel". Journal of the Taiwan Institute of Chemical Engineers. 92: 118–122. doi:10.1016/j.jtice.2018.02.017. S2CID 103246330.
- ^ Kuang, Xiao; Arıcan, Mehmet Onur; Zhou, Tao; Zhao, Xuanhe; Zhang, Yu Shrike (2023-02-24). "Functional Tough Hydrogels: Design, Processing, and Biomedical Applications". Accounts of Materials Research. 4 (2): 101–114. doi:10.1021/accountsmr.2c00026. ISSN 2643-6728.
- ^ a b c Nian, Guodong; Kim, Junsoo; Bao, Xianyang; Suo, Zhigang (2022-09-20). "Making Highly Elastic and Tough Hydrogels from Doughs". Advanced Materials. 34 (50). Bibcode:2022AdM....3406577N. doi:10.1002/adma.202206577. ISSN 0935-9648. PMID 36126085 – via Wiley Online Library.
- ^ Xu, Shuai; Zhou, Zidi; Liu, Zishun; Sharma, Pradeep (2023-01-06). "Concurrent stiffening and softening in hydrogels under dehydration". Science Advances. 9 (1): eade3240. Bibcode:2023SciA....9E3240X. doi:10.1126/sciadv.ade3240. ISSN 2375-2548. PMC 9812377. PMID 36598986.
- ^ Kessler, Michael; Yuan, Tianyu; Kolinski, John M.; Amstad, Esther (2023-02-21). "Influence of the Degree of Swelling on the Stiffness and Toughness of Microgel-Reinforced Hydrogels". Macromolecular Rapid Communications. 44 (16): e2200864. doi:10.1002/marc.202200864. ISSN 1022-1336. PMID 36809684.
- ^ Bai, Ruobing; Yang, Jiawei; Morelle, Xavier P.; Yang, Canhui; Suo, Zhigang (2018-03-20). "Fatigue Fracture of Self-Recovery Hydrogels". ACS Macro Letters. 7 (3): 312–317. doi:10.1021/acsmacrolett.8b00045. ISSN 2161-1653. PMID 35632906.
- ^ Zhu, Ruixin; Zhu, Dandan; Zheng, Zhen; Wang, Xinling (2024-02-13). "Tough double network hydrogels with rapid self-reinforcement and low hysteresis based on highly entangled networks". Nature Communications. 15 (1): 1344. Bibcode:2024NatCo..15.1344Z. doi:10.1038/s41467-024-45485-8. ISSN 2041-1723. PMC 10864390. PMID 38350981.
- ^ Zhang, Guogao; Steck, Jason; Kim, Junsoo; Ahn, Christine Heera; Suo, Zhigang (2023-06-30). "Hydrogels of arrested phase separation simultaneously achieve high strength and low hysteresis". Science Advances. 9 (26): eadh7742. Bibcode:2023SciA....9H7742Z. doi:10.1126/sciadv.adh7742. ISSN 2375-2548. PMC 10313164. PMID 37390216.
- ^ Hadjichristidis, Nikos; Gnanou, Yves; Matyjaszewski, Krzysztof; Muthukumar, Murugappan, eds. (2022-03-07). Macromolecular Engineering: From Precise Synthesis to Macroscopic Materials and Applications (1 ed.). Wiley. doi:10.1002/9783527815562.mme0043. ISBN 978-3-527-34455-0.
- ^ a b c d e f Qiu Y, Park K (December 2001). "Environment-sensitive hydrogels for drug delivery". Advanced Drug Delivery Reviews. 53 (3): 321–339. doi:10.1016/S0169-409X(01)00203-4. PMID 11744175.
- ^ Zaragoza J, Chang A, Asuri P (January 2017). "Effect of crosslinker length on the elastic and compression modulus of poly(acrylamide) nanocomposite hydrogels". Journal of Physics: Conference Series. 790 (1): 012037. Bibcode:2017JPhCS.790a2037Z. doi:10.1088/1742-6596/790/1/012037. ISSN 1742-6588.
- ^ Cidade MT, Ramos DJ, Santos J, et al. (April 2019). "Injectable Hydrogels Based on Pluronic/Water Systems Filled with Alginate Microparticles for Biomedical Applications". Materials. 12 (7): 1083. Bibcode:2019Mate...12.1083C. doi:10.3390/ma12071083. PMC 6479463. PMID 30986948.
- ^ Rose S, Prevoteau A, Elzière P, et al. (January 2014). "Nanoparticle solutions as adhesives for gels and biological tissues". Nature. 505 (7483): 382–385. Bibcode:2014Natur.505..382R. doi:10.1038/nature12806. PMID 24336207. S2CID 205236639.
- ^ Zaragoza J, Fukuoka S, Kraus M, et al. (October 2018). "Exploring the Role of Nanoparticles in Enhancing Mechanical Properties of Hydrogel Nanocomposites". Nanomaterials. 8 (11): 882. doi:10.3390/nano8110882. PMC 6265757. PMID 30380606.
- ^ Nguyen LH, Kudva AK, Saxena NS, Roy K (October 2011). "Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel". Biomaterials. 32 (29): 6946–6952. doi:10.1016/j.biomaterials.2011.06.014. PMID 21723599.
- ^ Hua M, Wu D, Wu S, et al. (March 2021). "4D Printable Tough and Thermoresponsive Hydrogels". ACS Applied Materials & Interfaces. 13 (11): 12689–12697. doi:10.1021/acsami.0c17532. PMID 33263991. S2CID 227258845.
- ^ a b Hua M, Wu S, Ma Y, et al. (February 2021). "Strong tough hydrogels via the synergy of freeze-casting and salting out". Nature. 590 (7847): 594–599. Bibcode:2021Natur.590..594H. doi:10.1038/s41586-021-03212-z. OSTI 1774154. PMID 33627812. S2CID 232048202.
- ^ Lai YC, Wilson AC, Zantos SG (2000). "Contact Lenses". Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. doi:10.1002/0471238961. ISBN 9780471484943.
- ^ Schmid, Julian; Armstrong, Tobias; Dickhardt, Fabian J.; Iqbal, SK Rameez; Schutzius, Thomas M. (2023-12-22). "Imparting scalephobicity with rational microtexturing of soft materials". Science Advances. 9 (51): eadj0324. Bibcode:2023SciA....9J.324S. doi:10.1126/sciadv.adj0324. ISSN 2375-2548. PMC 10732533. PMID 38117897.
- ^ Jeon, Dasom; Park, Jinwoo; Shin, Changhwan; Kim, Hyunwoo; Jang, Ji-Wook; Lee, Dong Woog; Ryu, Jungki (2020-04-10). "Superaerophobic hydrogels for enhanced electrochemical and photoelectrochemical hydrogen production". Science Advances. 6 (15): eaaz3944. Bibcode:2020SciA....6.3944J. doi:10.1126/sciadv.aaz3944. ISSN 2375-2548. PMC 7148083. PMID 32300656.
- ^ Bae, Misol; Kang, Yunseok; Lee, Dong Woog; Jeon, Dasom; Ryu, Jungki (August 2022). "Superaerophobic Polyethyleneimine Hydrogels for Improving Electrochemical Hydrogen Production by Promoting Bubble Detachment". Advanced Energy Materials. 12 (29): 2201452. Bibcode:2022AdEnM..1201452B. doi:10.1002/aenm.202201452. ISSN 1614-6832. S2CID 249355500.
- ^ Park, Jinwoo; Jeon, Dasom; Kang, Yunseok; Ryu, Jungki; Lee, Dong Woog (2023-01-24). "Nanofibrillar hydrogels outperform Pt/C for hydrogen evolution reactions under high-current conditions". Journal of Materials Chemistry A. 11 (4): 1658–1665. doi:10.1039/D2TA08775H. ISSN 2050-7496. S2CID 254387206.
- ^ Youhong Guo; H. Lu; F. Zhao; X. Zhou; W. Shi; Guihua Yu (2020). "Biomass-Derived Hybrid Hydrogel Evaporators for Cost-Effective Solar Water Purification". Advanced Materials. 32 (11): 1907061. Bibcode:2020AdM....3207061G. doi:10.1002/adma.201907061. PMID 32022974. S2CID 211036014.
- ^ Youhong Guo; C. M. Dundas; X. Zhou; K. P. Johnston; Guihua Yu (2021). "Molecular Engineering of Hydrogels for Rapid Water Disinfection and Sustainable Solar Vapor Generation". Advanced Materials. 33 (35): 2102994. Bibcode:2021AdM....3302994G. doi:10.1002/adma.202102994. PMID 34292641. S2CID 236174198.
- ^ Youhong Guo; W. Guan; C. Lei; H. Lu; W. Shi; Guihua Yu (2022). "Scalable super hygroscopic polymer films for sustainable moisture harvesting in arid environments". Nature Communications. 13 (1): 2761. Bibcode:2022NatCo..13.2761G. doi:10.1038/s41467-022-30505-2. PMC 9120194. PMID 35589809.
- ^ Brudno Y, Mooney DJ (December 2015). "On-demand drug delivery from local depots". Journal of Controlled Release. 219: 8–17. doi:10.1016/j.jconrel.2015.09.011. PMID 26374941.
- ^ Blacklow SO, Li J, Freedman BR, et al. (July 2019). "Bioinspired mechanically active adhesive dressings to accelerate wound closure". Science Advances. 5 (7): eaaw3963. Bibcode:2019SciA....5.3963B. doi:10.1126/sciadv.aaw3963. PMC 6656537. PMID 31355332.
- ^ Bordbar-Khiabani A, Gasik M (2022). "Smart hydrogels for advanced drug delivery systems". International Journal of Molecular Sciences. 23 (7): 3665. doi:10.3390/ijms23073665. PMC 8998863. PMID 35409025.
- ^ Jeon D, Park J, Shin C, et al. (April 2020). "Superaerophobic hydrogels for enhanced electrochemical and photoelectrochemical hydrogen production". Science Advances. 6 (15): eaaz3944. Bibcode:2020SciA....6.3944J. doi:10.1126/sciadv.aaz3944. PMC 7148083. PMID 32300656.
- ^ Discher DE, Janmey P, Wang YL (November 2005). "Tissue cells feel and respond to the stiffness of their substrate". Science. 310 (5751): 1139–1143. Bibcode:2005Sci...310.1139D. CiteSeerX 10.1.1.318.690. doi:10.1126/science.1116995. PMID 16293750. S2CID 9036803.
- ^ Schneider HJ, ed. (2015). Chemoresponsive Materials. Cambridge: Royal Society of Chemistry. ISBN 978-1-78262-242-0. Archived from the original on 2017-10-29. Retrieved 2019-04-17.
- ^ Yetisen AK, Naydenova I, da Cruz Vasconcellos F, et al. (October 2014). "Holographic sensors: three-dimensional analyte-sensitive nanostructures and their applications". Chemical Reviews. 114 (20): 10654–10696. doi:10.1021/cr500116a. PMID 25211200.
- ^ a b Lee JH (December 2018). "Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering". Biomaterials Research. 22 (1): 27. doi:10.1186/s40824-018-0138-6. PMC 6158836. PMID 30275970.
- ^ a b Liu M, Zeng X, Ma C, et al. (December 2017). "Injectable hydrogels for cartilage and bone tissue engineering". Bone Research. 5 (1): 17014. doi:10.1038/boneres.2017.14. PMC 5448314. PMID 28584674.
- ^ a b Pupkaite J, Rosenquist J, Hilborn J, Samanta A (September 2019). "Injectable Shape-Holding Collagen Hydrogel for Cell Encapsulation and Delivery Cross-linked Using Thiol-Michael Addition Click Reaction". Biomacromolecules. 20 (9): 3475–3484. doi:10.1021/acs.biomac.9b00769. PMID 31408340. S2CID 199574808.
- ^ a b Bertsch, Pascal; Diba, Mani; Mooney, David J.; Leeuwenburgh, Sander C. G. (25 January 2023). "Self-Healing Injectable Hydrogels for Tissue Regeneration". Chemical Reviews. 123 (2): 834–873. doi:10.1021/acs.chemrev.2c00179. PMC 9881015. PMID 35930422.
- ^ Mellati A, Dai S, Bi J, et al. (2014). "A biodegradable thermosensitive hydrogel with tuneable properties for mimicking three-dimensional microenvironments of stem cells". RSC Adv. 4 (109): 63951–63961. Bibcode:2014RSCAd...463951M. doi:10.1039/C4RA12215A. ISSN 2046-2069.
- ^ Malmsten M, Bysell H, Hansson P (2010-12-01). "Biomacromolecules in microgels — Opportunities and challenges for drug delivery". Current Opinion in Colloid & Interface Science. 15 (6): 435–444. doi:10.1016/j.cocis.2010.05.016. ISSN 1359-0294.
- ^ Nilsson, Peter; Hansson, Per (2005-12-01). "Ion-Exchange Controls the Kinetics of Deswelling of Polyelectrolyte Microgels in Solutions of Oppositely Charged Surfactant". The Journal of Physical Chemistry B. 109 (50): 23843–23856. doi:10.1021/jp054835d. ISSN 1520-6106. PMID 16375370.
- ^ Wanselius, Marcus; Rodler, Agnes; Searle, Sean S.; Abrahmsén-Alami, Susanna; Hansson, Per (2022-09-15). "Responsive Hyaluronic Acid–Ethylacrylamide Microgels Fabricated Using Microfluidics Technique". Gels. 8 (9): 588. doi:10.3390/gels8090588. ISSN 2310-2861. PMC 9498840. PMID 36135299.
- ^ Wanselius, Marcus; Searle, Sean; Rodler, Agnes; Tenje, Maria; Abrahmsén-Alami, Susanna; Hansson, Per (June 2022). "Microfluidics platform for studies of peptide – polyelectrolyte interaction". International Journal of Pharmaceutics. 621: 121785. doi:10.1016/j.ijpharm.2022.121785. ISSN 0378-5173. PMID 35500690.
- ^ Irving, Michael (2022-08-31). "Hydrogel glass windows let in more light and less heat". New Atlas. Retrieved 2022-09-26.
- ^ Miller, Brittney J. (8 June 2022). "How smart windows save energy". Knowable Magazine. doi:10.1146/knowable-060822-3. Retrieved 15 July 2022.
- ^ "A new way to cool down electronic devices, recover waste heat". Phys.org. April 22, 2020. Retrieved April 23, 2020.
- ^ Lavars, Nick (2022-12-15). "New protein-based armor material can withstand supersonic impacts". New Atlas. Retrieved 2022-12-25.
- ^ Strong, Vincent; Holderbaum, William; Hayashi, Yoshikatsu (September 18, 2024). "Electro-active polymer hydrogels exhibit emergent memory when embodied in a simulated game environment". Cell Reports Physical Science. doi:10.1016/j.xcrp.2024.102151.
- ^ Tang Y, Heaysman CL, Willis S, Lewis AL (September 2011). "Physical hydrogels with self-assembled nanostructures as drug delivery systems". Expert Opinion on Drug Delivery. 8 (9): 1141–1159. doi:10.1517/17425247.2011.588205. PMID 21619469. S2CID 24843309.
- ^ Aurand ER, Lampe KJ, Bjugstad KB (March 2012). "Defining and designing polymers and hydrogels for neural tissue engineering". Neuroscience Research. 72 (3): 199–213. doi:10.1016/j.neures.2011.12.005. PMC 3408056. PMID 22192467.
- ^ Ozcelik B, Brown KD, Blencowe A, et al. (May 2013). "Ultrathin chitosan-poly(ethylene glycol) hydrogel films for corneal tissue engineering". Acta Biomaterialia. 9 (5): 6594–6605. doi:10.1016/j.actbio.2013.01.020. PMID 23376126.
- ^ Gao J, Liu R, Wu J, et al. (May 2012). "The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury". Biomaterials. 33 (14): 3673–3681. doi:10.1016/j.biomaterials.2012.01.061. PMID 22361096.
- ^ Otani Y, Tabata Y, Ikada Y (April 1999). "Sealing effect of rapidly curable gelatin-poly (L-glutamic acid) hydrogel glue on lung air leak". The Annals of Thoracic Surgery. 67 (4): 922–926. doi:10.1016/S0003-4975(99)00153-8. PMID 10320229.
- ^ Ramdas M, Dileep KJ, Anitha Y, et al. (April 1999). "Alginate encapsulated bioadhesive chitosan microspheres for intestinal drug delivery". Journal of Biomaterials Applications. 13 (4): 290–296. doi:10.1177/088532829901300402. PMID 10340211. S2CID 31364133.
- ^ Liu X, Ma L, Mao Z, Gao C (2011), Jayakumar R, Prabaharan M, Muzzarelli RA (eds.), "Chitosan-Based Biomaterials for Tissue Repair and Regeneration", Chitosan for Biomaterials II, Advances in Polymer Science, vol. 244, Springer Berlin Heidelberg, pp. 81–127, doi:10.1007/12_2011_118, ISBN 978-3-642-24061-4
- ^ Wu ZL, Gong JP (June 2011). "Hydrogels with self-assembling ordered structures and their functions". NPG Asia Materials. 3 (6): 57–64. doi:10.1038/asiamat.2010.200. ISSN 1884-4057.
- ^ Kim J, Yaszemski MJ, Lu L (December 2009). "Three-dimensional porous biodegradable polymeric scaffolds fabricated with biodegradable hydrogel porogens". Tissue Engineering. Part C, Methods. 15 (4): 583–594. doi:10.1089/ten.TEC.2008.0642. PMC 2819712. PMID 19216632.
- ^ Cook MT, Smith SL, Khutoryanskiy VV (October 2015). "Novel glycopolymer hydrogels as mucosa-mimetic materials to reduce animal testing". Chemical Communications. 51 (77): 14447–14450. doi:10.1039/C5CC02428E. hdl:2299/16512. PMID 26221632.
- ^ Cook MT, Khutoryanskiy VV (November 2015). "Mucoadhesion and mucosa-mimetic materials--A mini-review". International Journal of Pharmaceutics. 495 (2): 991–998. doi:10.1016/j.ijpharm.2015.09.064. hdl:2299/16856. PMID 26440734.
Further reading
[edit]- Warren DS, Sutherland SP, Kao JY, et al. (2017). "The Preparation and Simple Analysis of a Clay Nanoparticle Composite Hydrogel". Journal of Chemical Education. 94 (11): 1772–1779. Bibcode:2017JChEd..94.1772W. doi:10.1021/acs.jchemed.6b00389. ISSN 0021-9584.











