Sulfolobus: Difference between revisions
Bongwarrior (talk | contribs) m Reverted edits by 72.28.2.194 (talk) to last version by Rhinestone K |
|||
| Line 84: | Line 84: | ||
== Ecology == |
== Ecology == |
||
S. solfataricus has been found in different areas including Yellowstone National Park, Mount St. Helens, Iceland, Italy, and Russia to name a few. Sulfolobus is located almost wherever there is volcanic activity. They thrive in environments where the temperature is about 80°C |
S. solfataricus has been found in different areas including Yellowstone National Park, Mount St. Helens, Iceland, Italy, and Russia to name a few. Sulfolobus is located almost wherever there is volcanic activity. They thrive in environments where the temperature is about 80°C with a pH at about 3 and sulfur present. Another species, S. tokodaii, has been located in an acidic spa in Beppu Hot Springs, Kyushu, Japan. Sediments from ~90m below the seafloor on the Peruvian continental margin are dominated by intact archaeal tetraethers, and a significant fraction of the community is sedimentary archaea taxonomically linked to the crenarchaeal Sulfolobales (Sturt, ''et al.'', 2004). |
||
==DNA damage response== |
==DNA damage response== |
||
Revision as of 17:45, 13 November 2013
| Sulfolobus | |
|---|---|
| |
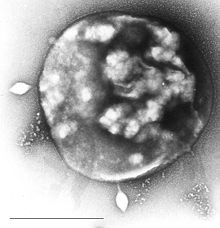
| Electron micrograph of Sulfolobus infected with Sulfolobus virus STSV1. Bar = 1 μm. | |
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Sulfolobus Brock, Brock, Belly & Weiss, 1972
|
| Species | |
| |
Sulfolobus is a genus of microorganism in the family Sulfolobaceae. It belongs to the archaea domain.[1]
Sulfolobus species grow in volcanic springs with optimal growth occurring at pH 2-3 and temperatures of 75-80 °C, making them acidophiles and thermophiles respectively. Sulfolobus cells are irregularly shaped and flagellar.
Species of Sulfolobus are generally named after the location from which they were first isolated, e.g. Sulfolobus solfataricus was first isolated in the Solfatara volcano. Other species can be found throughout the world in areas of volcanic or geothermal activity, such as geological formations called mud pots, which are also known as solfatare (plural of solfatara).
Sulfolobus as a model to study the molecular mechanisms of DNA replication
As the first Archaeal genome, Methanococcus jannaschii, has been sequenced completely, in 1996, it was found that the genes in the genome of Methanococcus jannaschii involved in DNA replication, transcription, translation, were more related to those counterparts in eukaryotes than in prokaryotes. In 2001, the first genome sequence of Sulfolobus, Sulfolobus solfataricus P2, was published. In P2's genome, the genes related to chromosome replication were found to be more related to eukaryotes. These genes include DNA polymerase, primase (including two subunits), MCM, CDC6/ORC1, RPA, RPC, PCNA. In 2004, the origins of DNA replication of Sulfolobus solfataricus and Sulfolobus acidocaldarius were identified. It showed that both species contained 2 origins in their genome. This is the first time that more than a single origin of DNA replication has been shown to be used in a prokaryotic cell. The mechanism of DNA replication in archaea is evolutionary conserved, and similar to that of eukaryotes. Sulfolobus is now used as a model to study the molecular mechanisms of DNA replication in Archaea. And because the system of DNA replication in Archaea is much simpler than that in Eukaryota, it was suggested that Archaea could be used as model to study the much more complexed DNA replication in Eukaryota.
Role in biotechnology
Sulfolobus proteins are of interest for biotechnology and industrial use due to their thermostable nature. One application is the creation of artificial derivatives from S. acidocaldarius proteins, named affitins. Intracellular proteins are not necessarily stable at low pH however, as Sulfolobus species maintain a significant pH gradient across the outer membrane. Sulfolobales are metabolically dependent on sulfur: heterotrophic or autotrophic, their energy comes from the oxidation of sulfur and/or cellular respiration in which sulfur acts as the final electron acceptor. For example, S. tokodaii is known to oxidize hydrogen sulfide to sulfate intracellularly.
Sulfolobus as a viral host
Lysogenic viruses infect Sulfolobus for protection. The viruses cannot survive in the extremely acidic and hot conditions that Sulfolobus lives in, and so the viruses use Sulfolobus as protection against the harsh elements. This relationship allows the virus to replicate inside the archaea without being destroyed by the environment.
Genome status
The complete genomes have been sequenced for S. acidocaldarius DSM 639 (2,225,959 nucleotides), S. solfataricus P2 (2,992,245 nucleotides), and S. tokodaii str. 7 (2,694,756 nucleotides).
Genome structure
The archaeon Sulfolobus solfataricus has a circular chromosome that consists of 2,992,245 bp. Another sequenced species, S. tokodaii has a circular chromosome as well but is slightly smaller with 2,694,756 bp. Both species lack the genes ftsZ and minD, which has been characteristic of sequenced crenarchaeota. They also code for citrate synthase and two subunits of 2-oxoacid:ferredoxin oxidoreductase, which plays the same role as alpha-ketoglutarate dehydrogenase in the TCA cycle. This indicates that Sulfolobus has a TCA cycle system similar to that found in mitochondria of eukaryotes. Other genes in the respiratory chain which partake in the production of ATP were not similar to what is found in eukaryotes. Cytochrome c is one such example that plays an important role in electron transfer to oxygen in eukaryotes. This was also found in A. pernix K1. Since this step is important for an aerobic microorganism like Sulfolobus, it probably uses a different molecule for the same function or has a different pathway.
Cell structure and metabolism
Sulfolobus can grow either lithoautotrophically by oxidizing sulfur, or chemoheterotrophically using sulfur to oxidize simple reduced carbon compounds. Heterotrophic growth has only been observed, however, in the presence of oxygen. The principle metabolic pathways are a glycolytic pathway, a pentose phosphate pathway, and the TCA cycle.
All Archaea have lipids with ether links between the head group and side chains, making the lipids more resistant to heat and acidity than bacterial and eukaryotic ether-linked lipids. The Sulfolobales are known for unusual tetraether lipids.In Sulfolobales, the ether-linked lipids are joined covalently across the "bilayer," making tetraethers. Technically, therefore, the tetraethers form a monolayer, not a bilayer. The tetraethers help Sulfolobus species survive extreme acid as well as high temperature.
Ecology
S. solfataricus has been found in different areas including Yellowstone National Park, Mount St. Helens, Iceland, Italy, and Russia to name a few. Sulfolobus is located almost wherever there is volcanic activity. They thrive in environments where the temperature is about 80°C with a pH at about 3 and sulfur present. Another species, S. tokodaii, has been located in an acidic spa in Beppu Hot Springs, Kyushu, Japan. Sediments from ~90m below the seafloor on the Peruvian continental margin are dominated by intact archaeal tetraethers, and a significant fraction of the community is sedimentary archaea taxonomically linked to the crenarchaeal Sulfolobales (Sturt, et al., 2004).
DNA damage response
Exposure of Sulfolobus solfataricus to the DNA damaging agents UV-irradiation, bleomycin or mitomycin C induced cellular aggregation.[2] Other physical stressors, such as pH or temperature shift, did not induce aggregation, suggesting that induction of aggregation is caused specifically by DNA damage. Ajon et al.[2] showed that UV-induced cellular aggregation mediates chromosomal marker exchange with high frequency. Recombination rates exceeded those of uninduced cultures by up to three orders of magnitude. Frols et al.[3][4] and Ajon et al.[2] hypothesized that the UV-inducible DNA transfer process and subsequent homologous recombinational repair represents an important mechanism to maintain chromosome integrity. This response may be a primitive form of sexual interaction, similar to the more well-studied bacterial transformation that is also associated with DNA transfer between cells leading to homologous recombinational repair of DNA damage.[5][6][7] In another related species, Sulfolobus acidocaldarius, UV-irradiation also increased the frequency of recombination due to genetic exchange.[8]
Viruses
The Sulfolobus viruses are temperate or permanent lysogens. Permanent lysogens differ from lysogenic bacteriophages in that the host cells are not lysed after the induction of Fuselloviridae production and eventually return to the lysogenic state. They are also unique in the sense that the genes encoding the structural proteins of the virus are constantly transcribed and DNA replication appears to be induced. The viruses infecting archaea like Sulfolobus have to use a strategy to escape prolonged direct exposure to the type of environment their host lives in, which may explain some of their unique properties.
See also
References
- ^ See the NCBI webpage on Sulfolobus. Data extracted from the "NCBI taxonomy resources". National Center for Biotechnology Information. Retrieved 2007-03-19.
- ^ a b c Ajon M, Fröls S, van Wolferen M; et al. (2011). "UV-inducible DNA exchange in hyperthermophilic archaea mediated by type IV pili". Mol. Microbiol. 82 (4): 807–17. doi:10.1111/j.1365-2958.2011.07861.x. PMID 21999488.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Fröls S, Ajon M, Wagner M; et al. (2008). "UV-inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili formation". Mol. Microbiol. 70 (4): 938–52. doi:10.1111/j.1365-2958.2008.06459.x. PMID 18990182.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Fröls S, White MF, Schleper C (2009). "Reactions to UV damage in the model archaeon Sulfolobus solfataricus". Biochem. Soc. Trans. 37 (Pt 1): 36–41. doi:10.1042/BST0370036. PMID 19143598.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Gross J, Bhattacharya D (2010). "Uniting sex and eukaryote origins in an emerging oxygenic world". Biol. Direct. 5: 53. doi:10.1186/1745-6150-5-53. PMC 2933680. PMID 20731852.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Bernstein H, Bernstein C. (2010) Evolutionary Origin of Recombination during Meiosis. BioScience 60(7):498-505. doi: http://dx.doi.org/10.1525/bio.2010.60.7.5
- ^ Harris Bernstein, Carol Bernstein and Richard E. Michod (2011). Meiosis as an Evolutionary Adaptation for DNA Repair. Chapter 19 in DNA Repair. Inna Kruman editor. InTech Open Publisher. DOI: 10.5772/25117 http://www.intechopen.com/books/dna-repair/meiosis-as-an-evolutionary-adaptation-for-dna-repair
- ^ Wood ER, Ghané F, Grogan DW (1997). "Genetic responses of the thermophilic archaeon Sulfolobus acidocaldarius to short-wavelength UV light". J. Bacteriol. 179 (18): 5693–8. PMC 179455. PMID 9294423.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
- Madigan M; Martinko J (editors). (2005). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 0-13-144329-1.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link)
Further reading
Scientific journals
- Judicial Commission of the International Committee on Systematics of Prokaryotes (2005). "The nomenclatural types of the orders Acholeplasmatales, Halanaerobiales, Halobacteriales, Methanobacteriales, Methanococcales, Methanomicrobiales, Planctomycetales, Prochlorales, Sulfolobales, Thermococcales, Thermoproteales and Verrucomicrobiales are the genera Acholeplasma, Halanaerobium, Halobacterium, Methanobacterium, Methanococcus, Methanomicrobium, Planctomyces, Prochloron, Sulfolobus, Thermococcus, Thermoproteus and Verrucomicrobium, respectively. Opinion 79". Int. J. Syst. Evol. Microbiol. 55 (Pt 1): 517–518. doi:10.1099/ijs.0.63548-0. PMID 15653928.
- Brock TD, Brock KM, Belly RT, Weiss RL (1972). "Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature". Arch. Mikrobiol. 84 (1): 54–68. doi:10.1007/BF00408082. PMID 4559703.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Scientific books
- Stetter, KO (1989). "Order III. Sulfolobales ord. nov. Family Sulfolobaceae fam. nov.". In JT Staley, MP Bryant, N Pfennig, and JG Holt, eds. (ed.). Bergey's Manual of Systematic Bacteriology, Volume 3 (1st ed.). Baltimore: The Williams & Wilkins Co. p. 169.
{{cite book}}:|editor=has generic name (help)CS1 maint: multiple names: editors list (link)
