Rhodocene: Difference between revisions
m Date maintenance tags and general fixes: build 545: |
Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChemSpiderID InChI1 InChIKey1. |
||
| Line 13: | Line 13: | ||
| OtherNames = dicyclopentadienyl rhodium |
| OtherNames = dicyclopentadienyl rhodium |
||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| ChemSpiderID = 2339512 |
|||
| ⚫ | |||
| InChI1 = 1/2C5H5.Rh/c2*1-2-4-5-3-1;/h2*1-5H;/q2*-1;+2 |
|||
| InChIKey1 = IWAKCRNSZSPDTB-UHFFFAOYAC |
|||
| ⚫ | |||
| CASNo = 12318-21-7 |
| CASNo = 12318-21-7 |
||
| PubChem = |
| PubChem = |
||
Revision as of 16:17, 6 November 2010
| |
| Names | |
|---|---|
| IUPAC name
rhodocene, bis(η5-cyclopentadienyl)rhodium(II)
| |
| Other names
dicyclopentadienyl rhodium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H10Rh | |
| Molar mass | 233.095 g·mol−1 |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
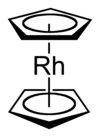
Rhodocene is an organometallic sandwich compound with the formula [Rh(C5H5)2]. Ferrocene was the first metallocene organometallic compound discovered[1] and it was demonstrated to have unusually high stability.[2] Soon after, analogous chemical structures with similarly high stability were reported for the unipositive cations of rhodocene and cobaltocene.[3] Aqueous solutions of the rhodicinium perchlorate salt [Rh(C5H5)2]ClO4 undergo one-electron reduction under polarographic conditions to form the neutral rhodocene species, but this could not be isolated.[3] Later research demonstrated that the paramagnetic 19-valence electron rhodocene radical is present at elevated temperatures in gas phase and when cooled to liquid nitrogen temperatures; however, at room temperature, rhodocene exists as a diamagnetic 18-valence electron ansa-metallocene dimer.[4][5][6] Just like ferrocene, the rhodocene monomer and rhodicinium cation adopt typical sandwich molecular structures, with two parallel cyclopentadienyl rings bound on opposite sides of a metal centre.[7] Owing to their stability and relative ease of preparation, 18-valence electron rhodicinium salts are the usual starting material for preparing rhodocene and substituted rhodocenes. The original synthesis made use of a Grignard carbanion and tris(acetylacetonato)rhodium(III)[3] and numerous other approaches have since been reported, including gas-phase redox transmetalation[8] and using half-sandwich precursors.[9] Octaphenylrhodocene was the first substituted rhodocene to be isolated at room temperature, though even it decomposes rapidly in air. X-ray crystallography confirmed that octaphenylrhodocene has a sandwich structure with a staggered conformation.[10]
History
Ferrocene was first synthesised in 1951 during an attempt to prepare the fulvalene bicyclopentadienylidene (C10H8) by oxidative coupling of cyclopentadiene; the resultant product was found to have molecular formula C10H10Fe and reported to exhibit "remarkable stability".[2] The discovery sparked substantial interest in the field of organometallic chemistry.[7][1] The initial challenge was to determine the structure of ferrocene in the hope of explaining its unexpected properties. The sandwich structure was deduced and reported independently by three groups in 1952: Robert Burns Woodward and Geoffrey Wilkinson investigated the reactivity in order to determine the structure,[11] Ernst Otto Fischer not only deduced the sandwich structure but also began synthesising other metallocenes including cobaltocene,[12] whilst the third group provided X-ray crystallographic confirmation of the sandwich structure.[13] The properties of cobaltocene reported by Wilkinson and Fischer demonstrated that the unipositive cobalticinium cation [Co(C5H5)2]+ exhibited stability similar to that of ferrocene itself, which led Wilkinson and F. Albert Cotton to attempt the synthesis of rhodicinium and iridicinium salts.[3] They reported the synthesis of numerous rhodicinium salts including the tribromide ([Rh(C5H5)2]Br3), perchlorate ([Rh(C5H5)2]ClO4), and reineckate ([Rh(C5H5)2][Cr(NCS)4(NH3)2]·H2O) anions, and found that the addition of dipicrylamine produced a compound of composition [Rh(C5H5)2][N(C6H2N3O6)2].[3] Polarographic investigation of rhodicinium perchlorate at neutral pH shows a cathodic wave peak at −1.53 V (versus SCE) at the dropping mercury electrode, corresponding to a one-election reduction forming rhodocene in solution; they were unable to isolate the neutral product from solution.[3] Wilkinson and Fischer went on to share the 1973 Nobel Prize for Chemistry for their "for their pioneering work, performed independently, on the chemistry of the organometallic, so called sandwich compounds".[14]
Structure
Organometallic compounds which obey the 18-electron rule generally display a high degree of stability; this explains the unusually high stability observed for ferrocene[2] and for the cobalticinium cation.[12] Just like the analogous compound cobaltocene, rhodocene has 19 valence electrons and thus is highly reactive, which explains early difficulties in isolating rhodocene from rhodicinium solutions.[3] Rhodocene exists as a paramagnetic radical monomer only at or below −196 °C (liquid nitrogen temperatures) or above 150 °C in gas phase.[4][5][6] It is this monomeric form that displays the typical staggered metallocene sandwich structure. At room temperature, the lifetime of the monomeric form in solution is less than two seconds;[6] instead, rhodocene exists as a diamagnetic bridged dimeric ansa-metallocene structure. Electron spin resonance, nuclear magnetic resonance and infrared spectroscopic measurements point to the presence of an equilibrium interconverting the monomeric and dimeric forms.[5] The dimerisation is a redox process, the dimer being a rhodium(I) species whilst the monomer has an rhodium(II) metal centre. Rhodium typically occupies oxidation states +I or +III in its stable compounds.[15]
The dimeric form is an example of a compound with related ligands of differing hapticity, the bridging ligand here being an η4-donor to each rhodium metal centre whilst the cyclopentadienyl anion remains an η5-donor. The consequence of the change in hapticity and oxidation state is that the dimeric form complies with the 18-electron rule, explaining both the diamagnetism of the dimeric form and its much higher stability.[5][6] Cotton and Wilkinson demonstrated[3] that the 18-electron rhodium(III) rhodicinium cation [Rh(C5H5)2]+ can be reduced in aqueous solution to the monomeric form; however, they were unable to isolate the neutral product as it is readily protonated to form the mixed-hapticity stable rhodium(I) species [(η5-C5H5)Rh(η4-C5H6)].[4] (η4-Cyclopentadiene)(η5-cyclopentadienyl)rhodium(I) is an unusual organometallic complex in that it has both a cyclopentadienyl anion and cyclopentadiene itself as ligands.
Synthesis
Rhodicinium salts were first reported[3] within two years of the discovery of ferrocene.[2] These salts were prepared by reacting the carbanion Grignard reagant cyclopentadienylmagnesium bromide (CpMgBr) with tris(acetylacetonato)rhodium(III) (Rh(acac)3). More recently, gas-phase rhodicinium cations have been generated by a redox transmetalation reaction of rhodium(I) ions with ferrocene or nickelocene.[8]
- Rh+ + [(η5-C5H5)2M] → M + [(η5-C5H5)2Rh]+ M = Ni or Fe
Modern microwave synthetic methods have also been reported.[16] Rhodicinium hexafluorophosphate forms after reaction of cyclopentadiene and rhodium(III) chloride hydrate in methanol following workup with methanolic ammonium hexafluorophosphate; the reaction yield exceeds 60% with only 30 seconds of microwaving.[17]
- RhCl3.xH2O + 2 C5H6 + NH4PF6 → [(η5-C5H5)2Rh]PF6 + 2 HCl + NH4Cl + xH2O
Rhodocene itself is then formed by reduction of rhodicinium salts with molten sodium.[4]
Substituted rhodocenes and rhodicinium salts
The body of knowledge concerning compounds with substituted cyclopentadienyl ligands is extensive with organometallic complexes of the pentamethylcyclopentadienyl and pentaphenylcyclopentadienyl ligands being well-known.[18] Known substituted rhodicinium salts include decamethylrhodicinium hexafluorophosphate [(η5-C5Me5)2Rh]PF6,[19] decaisopropylrhodicinium hexafluorophosphate [(η5-C5iPr5)2Rh]PF6,[20] and octaphenylrhodicinium hexafluorophosphate [(η5-C5Ph4H)2Rh]PF6.[10] Decaisopropylrhodicnium hexafluorophospate was synthesised in 1,2-dimethoxyethane in an unusual one-pot synthesis that involves the formation of 20 carbon-carbon bonds:
One-pot synthesis of decaisopropylrhodicinium hexafluorophosphate from decamethylrhodicinium hexafluorophosphate
In a similar reaction, pentaisopropylrhodicinium hexafluorophosphate [(η5-C5iPr5)Rh(η5-C5H5)]PF6 can be synthesised from pentamethylrhodicinium hexafluorophosphate [(η5-C5Me5)Rh(η5-C5H5)]PF6 in 80% yield.[20] These reactions demonstrate that the acidity of the methyl hydrogens in a pentamethylcyclopentadienyl complex can be considerably increased by the presence of the metal centre.

The compounds pentaphenylrhodicinium tetrafluoroborate [(η5-C5Ph5)Rh(η5-C5H5)]BF4, and pentamethylpentaphenylrhodicinium tetrafluoroborate [(η5-C5Ph5)Rh(η5-C5Me5)]BF4 have also been reported. They demonstrate that rhodium sandwich compounds can be prepared from half-sandwich precursors. Pentaphenylrhodicinium tetrafluoroborate, for example, has been synthesised from the tris(acetonitrile) salt [(η5-C5Ph5)Rh(CH3CN)3](BF4)2 by reaction with sodium cyclopentadienide:[9]
- [(η5-C5Ph5)Rh(MeCN)3](BF4)2 + C5H5− → [(η5-C5Ph5)Rh(η5-C5H5)]BF4 + BF4− + 3 MeCN
Octaphenylrhodocene, [(η5-C5Ph4H)2Rh], is the first rhodocene derivative to be isolated at room temperature. Its crystal structure shows a staggered conformation (similar to that of ferrocene, and in contrast to the eclipsed conformation of ruthenocene).[15] It decomposes rapidly in solution, and within minutes in air, demonstrating a dramatically greater air sensitivity than the analogous cobalt complex, although it is significantlt more stable than rhodocene itself. This difference is attributed to the relatively lower stability of the rhodium(II) state as compared to the cobalt(II) state. The synthesis of octaphenylrhodocene proceeds in three steps, with a diglyme reflux followed by workup with hexafluorophosphoric acid, then a sodium amalgam reduction in tetrahydrofuran:[10]
- Rh(acac)3 + 2 KC5Ph4H → [(η5-C5Ph4H)2Rh]+ + 2 K+ + 3 acac−
- [(η5-C5Ph4H)2Rh]+ + 3 acac− + 3 HPF6 → [(η5-C5Ph4H)2Rh]PF6 + 3 Hacac + 2 PF6−
- [(η5-C5Ph4H)2Rh]PF6 + Na/Hg → [(η5-C5Ph4H)2Rh] + NaPF6
Use of a rhodocene derivative in biomedical research

There has been substantial research using metallocene derivatives of ruthernium[21] and iron[22] as metallopharmaceuticals. One area of such research has utilised metallocenes in place of the fluorophenyl group in haloperidol.[23] The ferrocenyl-haloperidol compound investigated has structure (C5H5)Fe(C5H4)–C(=O)–(CH2)3–N(CH2CH2)2C(OH)–C6H4Cl and can be converted to the ruthenium analog via a transmetallation reaction. Using the radioactive isotope 103Ru produces a ruthenocenyl-haloperidol radiopharmaceutical with a high affinity for lung but not brain tissue in mice and rats.[23] Beta-decay of 103Ru produces the metastable isotope 103mRh in a rhodocenyl-haloperidol compound. This compound, like other rhodocene derivatives, has an unstable 19 valence electron configuration and rapidly oxidises to the expected cationic rhodocenium-haloperidol species.[23] The separation of the ruthenocenyl-haloperidol and the rhodocenium-haloperidol species and the distributions of each amongst bodily organs has been studied.[24]
References
- ^ a b Laszlo, P.; Hoffmann, R. (2000). "Ferrocene: Ironclad History or Rashomon Tale?". Angew. Chem. Int. Ed. 39: 123–124. doi:10.1002/(SICI)1521-3773(20000103)39:1<123::AID-ANIE123>3.0.CO;2-Z.
- ^ a b c d Kealy, T. J.; Pauson, P. L. (1951). "A New Type of Organo-Iron Compound". Nature. 168 (4285): 1039–1040. doi:10.1038/1681039b0.
- ^ a b c d e f g h i Cotton, F. A.; Whipple, R. O.; Wilkinson, G. (1953). "Bis-Cyclopentadienyl Compounds of Rhodium(III) and Iridium(III)". J. Am. Chem. Soc. 75 (14): 3586–3587. doi:10.1021/ja01110a504.
- ^ a b c d e Fischer, E. O.; Wawersik, H. (1966). "Uber Aromatenkomplexe von Metallen: LXXXVIII. Uber Monomeres und Dimeres Dicyclopentadienylrhodium und Dicyclopentadienyliridium und Uber Ein Neues Verfahren Zur Darstellung Ungeladener Metall-Aromaten-Komplexe". J. Organomet. Chem. (in German). 5 (6): 559–567. doi:10.1016/S0022-328X(00)85160-8.
- ^ a b c d Keller, H. J.; Wawersik, H. (1967). "Spectroscopic Investigations on Complex Compounds VI. EPR Spectra of (C5H5)2Rh AND (C5H5)2Ir". J. Organomet. Chem. (in German). 8 (1): 185–188. doi:10.1016/S0022-328X(00)84718-X.
- ^ a b c d El Murr, N.; Sheats, J. E.; Geiger, Jr., W. E.; Holloway, J. D. L. (1979). "Electrochemical Reduction Pathways of the Rhodocenium Ion. Dimerization and Reduction of Rhodocene". Inorg. Chem. 18 (6): 1443–1446. doi:10.1021/ic50196a007.
- ^ a b Federman Neto, A.; Pelegrino, A. C.; Darin, V. A. (2004). "Ferrocene: 50 Years of Transition Metal Organometallic Chemistry — From Organic and Inorganic to Supramolecular Chemistry". ChemInform. 35 (43). doi:10.1002/chin.200443242.
- ^ a b Jacobson, D. B.; Byrd, G. D.; Freiser, B. S. (1982). "Generation of Titanocene and Rhodocene Cations in the Gas Phase by a Novel Metal-Switching Reaction". J. Am. Chem. Soc. 104 (8): 2320–2321. doi:10.1021/ja00372a041.
- ^ a b He, T. (1999). Synthesis and Characterisation of Metallocenes Containing Bulky Cyclopentadienyl Ligands (PhD thesis). University of Sydney.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b c Collins, J. E.; Castellani, M. P.; Rheingold, A. L.; Miller, E. J.; Geiger, W. E.; Rieger, A. L.; Rieger, P. H. (1995). "Synthesis, Characterization, and Molecular-Structure of Bis(tetraphenylcyclopentadienyl)rhodium(II)". Organometallics. 14 (3): 1232–1238. doi:10.1021/om00003a025.
- ^ Wilkinson, G.; Rosenblum, M.; Whiting, M. C.; Woodward, R. B. (1952). "The Structure of Iron Bis-Cyclopentadienyl". J. Am. Chem. Soc. 74 (8): 2125–2126. doi:10.1021/ja01128a527.
- ^ a b Fischer, E. O.; Pfab, W. (1952). "Zur Kristallstruktur der Di-Cyclopentadienyl-Verbindungen des zweiwertigen Eisens, Kobalts und Nickels". Z. Naturforsch. B (in German). 7: 377–379.
- ^ Eiland, P. F.; Pepinsky, R. (1952). "X-ray Examination of Iron Biscyclopentadienyl". J. Am. Chem. Soc. 74 (19): 4971. doi:10.1021/ja01139a527.
- ^ "The Nobel Prize in Chemistry 1973". nobelprize.org. Retrieved 12 September 2010.
- ^ a b Cotton, F. A.; Wilkinson, G.; Murillo, C. A. (1999). "Part 3: The Chemistry of the Transition Elements". Advanced Inorganic Chemistry (6th ed.). New York: Wiley. ISBN 0471199575.
- ^ Baghurst, D. R.; Mingos, D. M. P. (1990). "Design and Application of a Reflux Modification for the Synthesis of Organometallic Compounds Using Microwave Dielectric Loss Heating Effects". J. Organomet. Chem. 384 (3): C57–C60. doi:10.1016/0022-328X(90)87135-Z.
- ^ Baghurst, D. R.; Mingos, D. M. P.; Watson, M. J. (1989). "Application of Microwave Dielectric Loss Heating Effects for the Rapid and Convenient Synthesis of Organometallic Compounds". J. Organomet. Chem. 368 (3): C43–C45. doi:10.1016/0022-328X(89)85418-X.
- ^ Okuda, J. (1992). "Transition-Metal Complexes of Sterically Demanding Cyclopentadienyl Ligands". Topics in Current Chemistry. 160: 97–145. doi:10.1007/3-540-54324-4_3.
- ^ Kolle, U.; Klaui, W. Z. (1991). "Preparation and Redox Behavior of a Series of Mixed-Ligand Cp* Aqua Tripod Complexes of Co, Rh and Ru". Z. Naturforsch. B. 46 (1): 75–83.
- ^ a b Buchholz, D.; Astruc, D. (1994). "The First Decaisopropylmetallocene - One-Pot Synthesis of [Rh(C5iPr5)2]PF6 from [Rh(C5Me5)2]PF6 by Formation of 20 Carbon-Carbon Bonds". Angew. Chem. Int. Ed. 33 (15–16): 1637–1639. doi:10.1002/anie.199416371.
- ^ Clarke, M. J. (2002). "Ruthenium Metallopharmaceuticals". Coord. Chem. Rev. 232 (1–2): 69–93. doi:10.1016/S0010-8545(02)00025-5.
- ^ Fouda, M. F. R.; Abd-Elzaher, M. M.; Abdelsamaia, R. A.; Labib, A. A. (2007). "On the Medicinal Chemistry of Ferrocene". Appl. Organomet. Chem. 21 (8): 613–625. doi:10.1002/aoc.1202.
- ^ a b c Wenzel, M.; Wu, Y. (1988). "Ferrocene, Ruthenocene, and Rhodocene Analogs of Haloperidol - Synthesis and Organ Distribution after Marking with 103Ru or 103mRh". Appl. Radiat. Isot. (in German). 39 (12): 1237–1241.
- ^ Wenzel, M.; Wu, Y. F. (1987). "Separation of 103mRh Rhodocene-Derivatives from their Parent 103Ru Ruthenocene-Derivatives and their Organ Distribution". Appl. Radiat. Isot. (in German). 38 (1): 67–69.
