Aufbau principle: Difference between revisions
mNo edit summary |
No edit summary |
||
| Line 16: | Line 16: | ||
== History == |
== History == |
||
[[Image:Sommerfeld ellipses.svg|thumb|Orbits with low angular momentum (''s''- and ''p''-orbitals) get closer to the nucleus]] |
[[Image:Sommerfeld ellipses.svg|thumb|Orbits with low angular momentum (''s''- and ''p''-orbitals) get closer to the nucleus]] |
||
The principle takes its name from the [[German language|German]] ''Aufbauprinzip'', "building-up principle", rather than being named for a scientist. In fact, it was formulated by |
The principle takes its name from the [[German language|German]] ''Aufbauprinzip'', "building-up principle", rather than being named for a scientist. In fact, it was formulated by Seimenns and Reuhrer. |
||
The Aufbau Principle states that: |
The Aufbau Principle states that: |
||
Revision as of 04:43, 14 May 2008
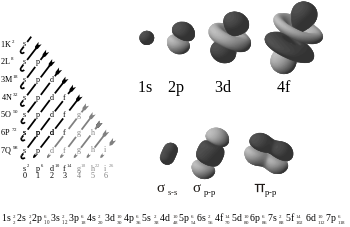
The Aufbau principle from German "Aufbau" meaning "construction" (also Aufbau rule or building-up principle), is used to determine the electron configuration of an atom, molecule or ion. The principle postulates a hypothetical process in which an atom is "built up" by progressively adding electrons. As they are added, they assume their most stable conditions (electron orbitals) with respect to the nucleus and those electrons already there.
According to the principle, electrons fill orbitals starting at the lowest available (possible) energy states before filling higher states (e.g. 1s before 2s). Orbitals are generally filled according to the n+l rule (also known as the Madelung rule after Erwin Madelung), where orbitals with a lower n+l value are filled before those with higher n+l values. The rule is based on the total number of nodes in the atomic orbital, n+l, which is related to the energy.[1] In case of equal n+l values, the orbital with a lower n value is filled first. Mathematically, the Aufbau principle and the Madelung rule can be described in terms of the tetrahedral sphere packing.
The number of electrons that can occupy each orbital is limited by the Pauli exclusion principle. If multiple orbitals of the same energy are available, Hund's rule says that unoccupied orbitals will be filled before occupied orbitals are reused (by electrons having different spins).
A version of the Aufbau principle can also be used to predict the configuration of protons and neutrons in an atomic nucleus.
Copper and chromium are common exceptions to the Aufbau principle:
Elemental copper should have 11 electrons in the outermost shell. But, its electronic configuration is [Ar].3d10.4s1 instead of [Ar].3d9.4s2 due to the greater stability of a half-filled or fully-filled orbital. Similarly, chromium takes the electronic configuration of [Ar].3d5.4s1 instead of [Ar].3d44s2.
History
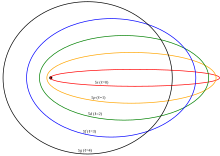
The principle takes its name from the German Aufbauprinzip, "building-up principle", rather than being named for a scientist. In fact, it was formulated by Seimenns and Reuhrer.
The Aufbau Principle states that:
- The orbitals of lower energy are filled in first with the electrons and only then the orbitals of high energy are filled.
It was an early application of quantum mechanics to the properties of electrons, and explained chemical properties in physical terms. Each added electron is subject to the electric field created by the positive charge of atomic nucleus and the negative charge of other electrons that are bound to the nucleus. Although in hydrogen there is no energy difference between orbitals with the same principal quantum number n, this is not true for the outer electrons of other atoms. Semiclassically, orbitals with the highest angular momentum are 'circular orbits' outside the inner electrons, but orbits with low angular momentum (s- and p-orbitals) have high orbital eccentricity, get closer to the nucleus and feel on average a less strongly screened nuclear charge. That explains why 4s-orbitals are filled before even 3d-orbitals.
References
- ^ Weinhold, F.; Landis, C. Valency and bonding, Cambridge, 2005; pp. 715-716. ISBN 0521831288.
