Hemoglobin: Difference between revisions
m →Quaternary structure: link to salt bridges |
No edit summary |
||
| (12 intermediate revisions by 9 users not shown) | |||
| Line 2: | Line 2: | ||
'''Hemoglobin''' or '''haemoglobin''' (frequently abbreviated as '''Hb''', {{PDB|1A3N}}) is the [[iron]]-containing [[oxygen]]-transport [[metalloprotein]] in the [[red blood cell|red cells]] of the [[blood]] in [[mammal]]s and other animals. Hemoglobin transports oxygen from the [[lung]]s to the rest of the body. In the [[tissue]]s, such as the [[muscle]], hemoglobin leaves its oxygen atoms and instead picks up the waste product [[carbon dioxide]], transporting it to the lungs where it can be exhaled. |
'''Hemoglobin''' or '''haemoglobin''' (frequently abbreviated as '''Hb''', {{PDB|1A3N}}) is the [[iron]]-containing [[oxygen]]-transport [[metalloprotein]] in the [[red blood cell|red cells]] of the [[blood]] in [[mammal]]s and other animals. Hemoglobin transports oxygen from the [[lung]]s to the rest of the body. In the [[tissue]]s, such as the [[muscle]], hemoglobin leaves its oxygen atoms and instead picks up the waste product [[carbon dioxide]], transporting it to the lungs where it can be exhaled. |
||
The name ''hemoglobin'' is the concatenation of ''heme'' and ''globin'', a [[globin]] being a generic term for a [[globular protein]]. Since a single [[subunit]] of hemoglobin is, in fact, made of a heme |
The name ''hemoglobin'' is the concatenation of ''heme'' and ''globin'', a [[globin]] being a generic term for a [[globular protein]]. Since a single [[subunit]] of hemoglobin is, in fact, made of a heme embedded in a globular protein, the name makes sense. The globin consists of four [[subunit]]s, which each bind a [[heme]] (or haem) groups. The heme groups are [[organic]] molecules, and containg one [[iron]] [[atom]] each that is important for the binding of oxygen to the molecule. Mutations in the [[gene]] for the haemoglobin protein result in a group of hereditary diseases termed the ''[[hemoglobinopathy|hemoglobinopathies]]'', the most common members of which are [[sickle-cell disease]] and [[thalassaemia]]. |
||
==Structure== |
==Structure== |
||
[[Image:Heme.png|right|framed|Heme group]] |
[[Image:Heme.png|right|framed|Heme group]] |
||
In each [[subunit]] of a |
In each [[subunit]] of a haemoglobin [[molecule]], there is a [[haem]] group. A haem group consists of an iron atom held in a [[heterocyclic]] ring, known as a ''[[porphyrin]]''. This iron atom is the site of oxygen binding. The iron atom binds equally to all four [[nitrogen]]s in the center of the ring, which lie in one plane. Two additional bonds perpendicular to the plane on each side can be formed with the iron to form the fifth and sixth positions. The iron atom can either be in the Fe<sup>+2</sup> or Fe<sup>+3</sup> state, but ferrihaemoglobin ([[Methaemoglobin]]) (Fe<sup>3+</sup>) cannot bind oxygen. All haemoglobin molecules contain either two α or two ξ chains. |
||
In adult humans, the most common |
In adult humans, the most common haemoglobin is a tetramer (contains 4 subunit proteins) called haemoglobin A, consisting of two α and two β subunits non-covalently bound. The subunits are structurally similar and about the same size. Each subunit has a molecular weight of about 16,000 [[dalton (unit)|dalton]]s, for a total [[molecular weight]] in the tetramer of about 64,000 daltons. Haemoglobin A is the most intensively studied of the haemoglobin molecules. |
||
===Quaternary structure=== |
===Quaternary structure=== |
||
| Line 15: | Line 16: | ||
===Types of hemoglobins=== |
===Types of hemoglobins=== |
||
Embryonic |
Embryonic haemoglobins: |
||
* Gower 1 (ξ<sub>2</sub>ε<sub>2</sub>) |
* Gower 1 (ξ<sub>2</sub>ε<sub>2</sub>) |
||
* Gower 2 (α<sub>2</sub>ε<sub>2</sub>) ({{PDB|1A9W}}) |
* Gower 2 (α<sub>2</sub>ε<sub>2</sub>) ({{PDB|1A9W}}) |
||
* |
* Haemoglobin Portland (ξ<sub>2</sub>γ<sub>2</sub>) |
||
Adult |
Adult haemoglobins (i.e. the haemoglobins that adults have as opposed to haemoglobins that only exist in the foetus): |
||
* [[ |
* [[Haemoglobin F]] (α<sub>2</sub>γ<sub>2</sub>) ({{PDB|1FDH}}) - In adults Haemoglobin F is restricted to a limited population of red cells called F cells. |
||
* |
* Haemoglobin A (α<sub>2</sub>β<sub>2</sub>) ({{PDB|1BZ0}}) |
||
* |
* Haemaglobin A<sub>2</sub> (α<sub>2</sub>δ<sub>2</sub>) - δ chain synthesis begins late in the third trimester and in adults, it has a normal level of 2.5% |
||
==Binding of ligands== |
==Binding of ligands== |
||
| Line 33: | Line 34: | ||
:CO<sub>2</sub> + H<sub>2</sub>O <-> HCO<sub>3</sub><sup>-</sup> + H<sup>+</sup> |
:CO<sub>2</sub> + H<sub>2</sub>O <-> HCO<sub>3</sub><sup>-</sup> + H<sup>+</sup> |
||
[[Image:Hb saturation curve.png|thumb|320px|Hemoglobin sigmoidal oxygen dissociation curve]] |
[[Image:Hb saturation curve.png|left|thumb|320px|Hemoglobin sigmoidal oxygen dissociation curve]] |
||
So blood with high carbon dioxide levels is also lower in [[pH]] (more [[acidic]]). Hemoglobin can bind [[proton]]s and carbon dioxide which causes a conformational change in the protein and facilitates the release of oxygen. Protons bind at various places along the protein and carbon dioxide binds at the [[alpha-amino group]] forming [[carbamate]]. Conversely, when the carbon dioxide levels in the blood decrease (i.e. around the lungs), carbon dioxide is released, increasing the oxygen affinity of the protein. This control of hemoglobin's affinity for oxygen by the binding and release of carbon dioxide is known as the [[Bohr effect]]. |
So blood with high carbon dioxide levels is also lower in [[pH]] (more [[acidic]]). Hemoglobin can bind [[proton]]s and carbon dioxide which causes a conformational change in the protein and facilitates the release of oxygen. Protons bind at various places along the protein and carbon dioxide binds at the [[alpha-amino group]] forming [[carbamate]]. Conversely, when the carbon dioxide levels in the blood decrease (i.e. around the lungs), carbon dioxide is released, increasing the oxygen affinity of the protein. This control of hemoglobin's affinity for oxygen by the binding and release of carbon dioxide is known as the [[Bohr effect]]. |
||
| Line 97: | Line 99: | ||
[[Category:Hematology]] |
[[Category:Hematology]] |
||
[[Category:Hemoproteins]] |
[[Category:Hemoproteins]] |
||
| ⚫ | |||
[[da:Hæmoglobin]] |
[[da:Hæmoglobin]] |
||
[[de:Hämoglobin]] |
[[de:Hämoglobin]] |
||
| ⚫ | |||
[[es:Hemoglobina]] |
[[es:Hemoglobina]] |
||
[[eo:Hemoglobino]] |
[[eo:Hemoglobino]] |
||
| Line 109: | Line 112: | ||
[[it:Emoglobina]] |
[[it:Emoglobina]] |
||
[[he:המוגלובין]] |
[[he:המוגלובין]] |
||
[[lt:Hemoglobinas]] |
|||
[[ms:Hemoglobin]] |
[[ms:Hemoglobin]] |
||
[[nl:Hemoglobine]] |
[[nl:Hemoglobine]] |
||
[[ja:ヘモグロビン]] |
[[ja:ヘモグロビン]] |
||
| ⚫ | |||
[[nn:Hemoglobin]] |
|||
[[pl:Hemoglobina]] |
[[pl:Hemoglobina]] |
||
[[pt:Hemoglobina]] |
[[pt:Hemoglobina]] |
||
[[ru:Гемоглобин]] |
[[ru:Гемоглобин]] |
||
[[sr:Хемоглобин]] |
[[sr:Хемоглобин]] |
||
| ⚫ | |||
[[fi:Hemoglobiini]] |
[[fi:Hemoglobiini]] |
||
[[sv:Hemoglobin]] |
|||
[[zh:血红蛋白]] |
[[zh:血红蛋白]] |
||
Revision as of 10:56, 15 November 2005
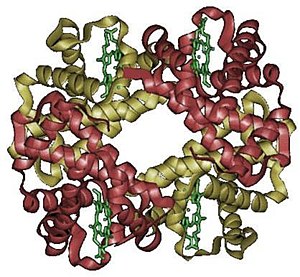
Hemoglobin or haemoglobin (frequently abbreviated as Hb, PDB: 1A3N) is the iron-containing oxygen-transport metalloprotein in the red cells of the blood in mammals and other animals. Hemoglobin transports oxygen from the lungs to the rest of the body. In the tissues, such as the muscle, hemoglobin leaves its oxygen atoms and instead picks up the waste product carbon dioxide, transporting it to the lungs where it can be exhaled.
The name hemoglobin is the concatenation of heme and globin, a globin being a generic term for a globular protein. Since a single subunit of hemoglobin is, in fact, made of a heme embedded in a globular protein, the name makes sense. The globin consists of four subunits, which each bind a heme (or haem) groups. The heme groups are organic molecules, and containg one iron atom each that is important for the binding of oxygen to the molecule. Mutations in the gene for the haemoglobin protein result in a group of hereditary diseases termed the hemoglobinopathies, the most common members of which are sickle-cell disease and thalassaemia.
Structure

In each subunit of a haemoglobin molecule, there is a haem group. A haem group consists of an iron atom held in a heterocyclic ring, known as a porphyrin. This iron atom is the site of oxygen binding. The iron atom binds equally to all four nitrogens in the center of the ring, which lie in one plane. Two additional bonds perpendicular to the plane on each side can be formed with the iron to form the fifth and sixth positions. The iron atom can either be in the Fe+2 or Fe+3 state, but ferrihaemoglobin (Methaemoglobin) (Fe3+) cannot bind oxygen. All haemoglobin molecules contain either two α or two ξ chains.
In adult humans, the most common haemoglobin is a tetramer (contains 4 subunit proteins) called haemoglobin A, consisting of two α and two β subunits non-covalently bound. The subunits are structurally similar and about the same size. Each subunit has a molecular weight of about 16,000 daltons, for a total molecular weight in the tetramer of about 64,000 daltons. Haemoglobin A is the most intensively studied of the haemoglobin molecules.
Quaternary structure
The four polypeptide chains are bound to each other by salt bridges, hydrogen bonds and hydrophobic interaction. There are two kinds of contacts between the α and β chains: α1β1 and α1β2.
Types of hemoglobins
Embryonic haemoglobins:
Adult haemoglobins (i.e. the haemoglobins that adults have as opposed to haemoglobins that only exist in the foetus):
- Haemoglobin F (α2γ2) (PDB: 1FDH) - In adults Haemoglobin F is restricted to a limited population of red cells called F cells.
- Haemoglobin A (α2β2) (PDB: 1BZ0)
- Haemaglobin A2 (α2δ2) - δ chain synthesis begins late in the third trimester and in adults, it has a normal level of 2.5%
Binding of ligands
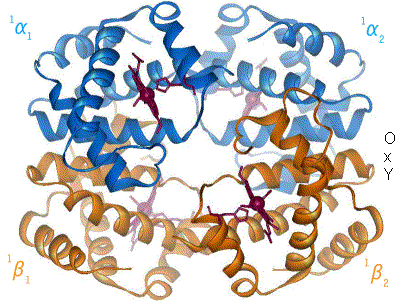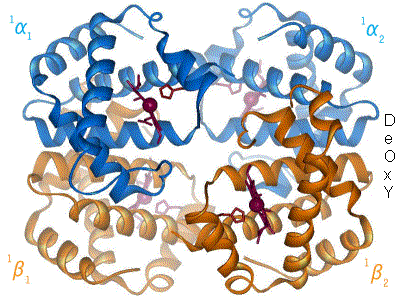
In the tetrameric form of normal adult hemoglobin, the binding of oxygen is a cooperative process. The binding affinity of hemoglobin for oxygen is increased by the oxygen saturation of the molecule. As a consequence, the oxygen binding curve of hemoglobin is sigmoidal, or 'S' shaped, as opposed to the normal hyperbolic (noncooperative) curve. This positive cooperative binding is achieved through steric conformational changes of the hemoglobin protein complex: when one subunit protein in hemoglobin becomes oxygenated it induces a confirmation or structural arrangement change in the whole complex causing the other 3 subunits to gain an increased affinity for oxygen.
Hemoglobin's affinity for oxygen is decreased in the presence of carbon monoxide because both gases compete for the same binding sites on hemoglobin, carbon monoxide binding preferentially to oxygen. Carbon dioxide occupies a different binding site on the hemoglobin. Carbon dioxide reacts with water to give bicarbonate, carbonic acid freed protons via the reaction, which is catalyzed by carbonic anhydrase:
- CO2 + H2O <-> HCO3- + H+

So blood with high carbon dioxide levels is also lower in pH (more acidic). Hemoglobin can bind protons and carbon dioxide which causes a conformational change in the protein and facilitates the release of oxygen. Protons bind at various places along the protein and carbon dioxide binds at the alpha-amino group forming carbamate. Conversely, when the carbon dioxide levels in the blood decrease (i.e. around the lungs), carbon dioxide is released, increasing the oxygen affinity of the protein. This control of hemoglobin's affinity for oxygen by the binding and release of carbon dioxide is known as the Bohr effect.
The binding of oxygen is affected by molecules such as carbon monoxide (CO) (For example from tobacco smoking, cars and furnaces). CO competes with oxygen at the heme binding site. Hemoglobin binding affinity for CO is 200 times greater than its affinity for oxygen, meaning that small amounts of CO dramatically reduces hemoglobin’s ability to transport oxygen. When hemoglobin combines with CO, it forms a very bright red compound called carboxyhemoglobin. When inspired air contains CO levels as low as 0.02% headache and nausea occur; if the CO concentration is increased to 0.1%, unconsciousness will follow. In heavy smokers, up to 20% of the oxygen active sites can be blocked by CO.
Hemoglobin also has competitive binding affinity for Sulfur monoxide (SO), Nitrogen Dioxide (NO2) and Hydrogen sulfide (H2S). The iron atom in the heme group must be in the Fe+2 oxidation state to support oxygen transport. Oxidation to Fe+3 state converts hemoglobin into hemiglobin or methemoglobin which cannot bind oxygen. Nitrogen dioxide and nitrous oxide are capable of converting hemoglobin to methemoglobin.
In people acclimated to high altitudes, the concentration of 2,3-diphosphoglycerate (2,3-DPG) in the blood is increased, which allows these individuals to deliver a larger amount of oxygen to tissues under conditions of lower oxygen tension. This phenomenon, where molecule Y affects the binding of molecule X to a transport molecule Z, is called a heterotropic allosteric effect.
A variant hemoglobin, called fetal hemoglobin (Hb F, α2γ2), is found in the developing fetus, and binds oxygen with greater affinity than adult hemoglobin. Consequently, the oxygen binding curve for fetal hemoglobin is left-shifted (i.e., a higher percentage of hemoglobin has oxygen bound to it at lower oxygen tension) in comparison to that of adult hemoglobin.
Degradation of hemoglobin
When red cells reach the end of their life, they are broken down, and the hemoglobin molecule broken up and the iron recycled. When the porphyrin ring is broken up, the fragments are normally secreted in the bile by the liver. The major final product of heme degradation is bilirubin. Increased levels of this chemical are detected in the blood if red cells are being destroyed more rapidly than usual. Improperly degraded hemoglobin protein or hemoglobin that has been released from the blood cells can clog small blood vessels especially the delicate blood filtering vessels of the kidneys, causing kidney damage.
Other biological oxygen-binding proteins
It should be noted that hemoglobin is by no means unique. There are a variety of oxygen transport and binding proteins throughout the animal (and plant) kingdom. Other organisms including bacteria, protozoans and fungi all have hemoglobin like proteins, their known and predicted roles include the reversible binding of gaseous ligands.
Myoglobin: Found in the muscle tissue of many vertebrates including humans (gives muscle tissue a distinct red or dark gray color). Is very similar to hemoglobin in structure and sequence, but is not arranged in tetramers, it is a monomer and lacks cooperative binding and is used to store oxygen rather than transport it.
Hemocyanin: Second most common oxygen transporting protein found in nature. Found in the blood of many arthropods and molluscs. Uses copper prosthetic group instead of iron heme groups and is blue in color when oxygenated.
Hemerythrin: Some marine invertebrates and a few species of annelid use this iron containing non-heme protein to carry oxygen in their blood. Appears pink/violet when oxygenated, clear when not.
Vanabins: also known as Vanadium Chromagen are found in the blood of Sea squirt and are hypothesised to use the rare metal Vanadium as its oxygen binding prosthetic group, but this hypothesis is unconfirmed.
Erythrocruorin: found in many annelids, including earthworms. Giant free-floating blood protein, contains many dozens even hundreds of Iron heme containing protein subunits bound together into a single protein complex with a molecular masses greater than 3.5 million daltons.
Pinnaglobin: Only seen in the mollusk Pinna squamosa. Brown manganese-based porphyrin protein.
Leghemoglobin: In leguminous plants, such as alfalfa or soybeans, the nitrogen fixing bacteria in the roots are protected from oxygen by this iron heme containing, oxygen binding protein.
Role in disease
Decreased levels of hemoglobin, with or without an absolute decrease of red blood cells, leads to symptoms of anemia. Anemia has many different causes, although iron deficiency and its resultant iron deficiency anemia are the most common causes in the Western world. As absence of iron decreases heme synthesis, red blood cells in iron deficiency anemia are hypochromic (lacking the red hemoglobin pigment) and microcytic (smaller than normal). Other anemias are rarer. In hemolysis (accelerated breakdown of red blood cells), associated jaundice is caused by the hemoglobin metabolite bilirubin, and the circulating hemoglobin can cause renal failure.
Mutations in the globin chain are associated with the haemoglobinopathies, such as Sickle-cell disease and thalassemia.
There is a group of genetic disorders, known as the porphyrias that are characterized by errors in metabolic pathways of heme synthesis. King George III of the United Kingdom was probably the most famous porphyria sufferer.
To a small extent, hemoglobin A slowly combines with glucose at a certain location in the molecule. The resulting molecule is often referred to as Hb A1c. As the concentration of glucose in the blood increases, the percentage of Hb A that turns into Hb A1c increases. In diabetics whose glucose usually runs high, the percent Hb A1c also runs high. Because of the slow rate of Hb A combination with glucose, the Hb A1c percentage is representative of glucose level in the blood averaged over a longer time (typically 3 months).
Diagnostic use
Hemoglobin levels are amongst the most commonly performed blood tests, usually as part of a full blood count. Results are reported in g/L, g/dl or mmol/L. For conversion, 1 g/dl is 0.62 mmol/l.
Glucose levels in blood can vary widely each hour, so one or only a few samples from a patient analyzed for glucose may not be representative of glucose control in the long run. For this reason a blood sample may be analyzed for Hb A1c, which is more representative of glucose control averaged over a longer time period. People whose Hb A1c runs 6.0% or less show good longer-term glucose control. Hb A1c values which are more than 7.0% are elevated. This test is especially useful for diabetics.
See also
- hemoprotein
- hemocyanin
- chlorophyll
- hemoglobin A1C
- hemoglobin S
- hemoglobin C
- hemoglobin F
- hemoglobin A2
