Heat: Difference between revisions
m Reverted edits by 204.218.240.33 (talk) to last version by Lateg |
No edit summary |
||
| Line 1: | Line 1: | ||
{{For| other uses|Heat (disambiguation)}} |
{{For| other uses|Heat (disambiguation)}} |
||
Heat is perceived as something which produces a sensation of warmth. The sensation will, of course, be stronger in higher temperatures. There cannot be any heat transfer between two objects that are at the same temperature. The amount of heat transferred depends on the temperature difference and the conductivity of the path between the two objects; the direction of heat flow is always toward the cooler object. |
|||
Earlier philosophers considered heat as a substance that was passed from hot objects to colder ones. This heat fluid was known as phlogiston (meaning flammable in Greek), which was thought to have a mass and the ability to flow in and out of objects during burning; it was considered to be the soul of matter. The concept was refined after Lavoisier an eighteenth century French chemist, showed that mass is a conserved quantity and does not change when a substance undergoes a chemical reaction. The new substance was called caloric, a mass-less fluid thought to flow from hot to cold objects. |
|||
It was not until the middle of the nineteenth century that Thompson and Joule showed that this theory is also wrong; heat is not a substance, but rather a manifestation of motion at the molecular level (kinetic theory). For example, when we rub our hands against each other both hands get warmer, even though initially they were at the same cooler temperatures. If the cause of the heat were a fluid, then it would have flowed from a (hotter) body with more energy to another with less energy (colder). Instead, the hands are heated because the kinetic energy of motion (rubbing) has been converted to heat in a process called “friction”. |
|||
The reason that the concept of heat as a fluid so well received for so many centuries was that it met all the human notions of how a fluid should behave. It could pass from one body to another, similarly to the flow of water in a river or a pipe. When a hot object comes into contact with a colder one, the hot object cools and the cold one gets hotter as if some substance were passing from one to the other. Heat is also capable of running a steam engine in the same manner that water could turn a waterwheel. |
|||
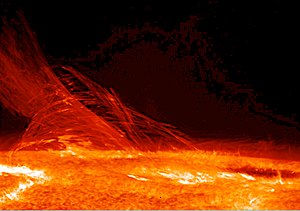
[[Image:171879main LimbFlareJan12 lg.jpg|300px|thumb|right|'''Heat''' generated from the electromagnetic radiation from the [[Sun]] is one of the driving [[force]]s of [[life]] on [[Earth]].]] |
[[Image:171879main LimbFlareJan12 lg.jpg|300px|thumb|right|'''Heat''' generated from the electromagnetic radiation from the [[Sun]] is one of the driving [[force]]s of [[life]] on [[Earth]].]] |
||
| Line 166: | Line 174: | ||
==External links== |
==External links== |
||
*[https://www.thermalfluidscentral.org/e-encyclopedia/index.php/Heat Heat] - article at Thermal-FluidsPedia. |
|||
*[http://www.foxnews.com/story/0,2933,187464,00.html Plasma heat at 2 gigakelvins] - Article about extremely high temperature generated by scientists (Foxnews.com) |
*[http://www.foxnews.com/story/0,2933,187464,00.html Plasma heat at 2 gigakelvins] - Article about extremely high temperature generated by scientists (Foxnews.com) |
||
*[http://www.cheresources.com/convection.shtml Correlations for Convective Heat Transfer] - ChE Online Resources |
*[http://www.cheresources.com/convection.shtml Correlations for Convective Heat Transfer] - ChE Online Resources |
||
Revision as of 15:37, 28 November 2010
Heat is perceived as something which produces a sensation of warmth. The sensation will, of course, be stronger in higher temperatures. There cannot be any heat transfer between two objects that are at the same temperature. The amount of heat transferred depends on the temperature difference and the conductivity of the path between the two objects; the direction of heat flow is always toward the cooler object.
Earlier philosophers considered heat as a substance that was passed from hot objects to colder ones. This heat fluid was known as phlogiston (meaning flammable in Greek), which was thought to have a mass and the ability to flow in and out of objects during burning; it was considered to be the soul of matter. The concept was refined after Lavoisier an eighteenth century French chemist, showed that mass is a conserved quantity and does not change when a substance undergoes a chemical reaction. The new substance was called caloric, a mass-less fluid thought to flow from hot to cold objects.
It was not until the middle of the nineteenth century that Thompson and Joule showed that this theory is also wrong; heat is not a substance, but rather a manifestation of motion at the molecular level (kinetic theory). For example, when we rub our hands against each other both hands get warmer, even though initially they were at the same cooler temperatures. If the cause of the heat were a fluid, then it would have flowed from a (hotter) body with more energy to another with less energy (colder). Instead, the hands are heated because the kinetic energy of motion (rubbing) has been converted to heat in a process called “friction”.
The reason that the concept of heat as a fluid so well received for so many centuries was that it met all the human notions of how a fluid should behave. It could pass from one body to another, similarly to the flow of water in a river or a pipe. When a hot object comes into contact with a colder one, the hot object cools and the cold one gets hotter as if some substance were passing from one to the other. Heat is also capable of running a steam engine in the same manner that water could turn a waterwheel.
In physics and thermodynamics, heat is energy transferred from one body or thermodynamic system to another due to thermal contact when the systems are at different temperatures. It is also often described as the process of transfer of energy between physical entities. In this description, it is an energy transfer to the body in any other way than due to work performed on the body.[1]
In engineering, energy transfer by heat between objects is classified as either thermal conduction, first described scientifically by Joseph Fourier, by fluid convection, which is the mixing of hot and cold fluid regions due to pressure differentials, and by thermal radiation, the transmission of electromagnetic radiation described by black body theory.
Thermodynamically, energy can only be transferred by heat between objects, or regions within an object, with different temperatures, a consequence of the zeroth law of thermodynamics. This transfer happens spontaneously only in the direction to the colder body, as per the second law of thermodynamics. The transfer of energy by heat from one object to another object with an equal or higher temperature can happen only with the aid of a heat pump via mechanical work or by using mirrors or lenses to focus electromagnetic radiation which thereby increase its energy flux density.
A related term is thermal energy, loosely defined as the energy of a body that increases with its temperature. Heat is also often referred to as thermal energy, although many definitions require this thermal energy to be in transfer between two systems to be called heat, otherwise, many sources prefer to continue to refer to the internal quantity as thermal energy.
Overview

Heat is defined as thermal energy in transit. Scottish physicist James Clerk Maxwell, in his 1871 classic Theory of Heat, was one of the first to enunciate a modern definition of heat. Maxwell outlined four stipulations for the definition of heat:
- It is something which may be transferred from one body to another, according to the second law of thermodynamics.
- It is a measurable quantity, and thus treated mathematically.
- It cannot be treated as a substance, because it may be transformed into something that is not a substance, e.g., mechanical work.
- It is one of the forms of energy.
Heat flows between systems that are not in thermal equilibrium with each other. It flows spontaneously from the areas of high temperature to areas of lower temperature. When two bodies of different temperature come into thermal contact, they exchange thermal energy, i.e. heat, until their temperatures are equal, that is until they reach thermal equilibrium.
The first law of thermodynamics states that the energy of an isolated system is conserved. Therefore, to change the energy of a system, energy must be transferred to or from the system. Heat and work are the only two mechanisms by which energy can be transferred. Work performed on a body is, by definition [1], an energy transfer to the body that is due to a change to external parameters of the body, such as the volume, magnetization, center of mass in a gravitational field. Heat is the energy transferred to the body in any other way.
In the case of bodies close to thermal equilibrium where notions such as the temperature can be defined, heat transfer can be related to temperature difference between bodies. It is an irreversible process that leads to the bodies coming closer to mutual thermal equilibrium.
Human notions such as hot and cold are relative terms and are generally used to compare one object’s temperature to another or its surroundings.
Definitions
Several modern definitions of heat are as follows:
- The energy transferred from a high-temperature object to a lower-temperature object is called heat.[2]
- Any spontaneous flow of energy from one object to another caused by a difference in temperature between the objects is called heat.[3]
- In a thermodynamic sense, heat is never regarded as being stored within a body. Like work, it exists only as energy in transit from one body to another or between a system and its surroundings. When energy in the form of heat is added to a system, it is stored as kinetic and potential energy of the atoms and molecules making up the system.[4]
Notation and units
The unit for the amount of energy transferred by heat in the International System of Units (SI) is the joule (J), though the British Thermal Unit (BTU) and the calorie are still used in the United States. The unit for the rate of heat transfer is the watt (W = J/s).
The total amount of energy transferred as heat is conventionally abbreviated as Q. The sign convention is that when a body releases heat into its surroundings, Q < 0 (negative); when a body absorbs heat from its surroundings, Q > 0 (positive). Heat transfer rate, or heat flow per unit time, is denoted by:
- .
It is often measured in watts. Heat flux is defined as rate of heat transfer per unit cross-sectional area, and is denotedq, resulting in units of watts per square metre.
Internal energy
The first law of thermodynamics states that the change in internal energy (U) of a system is given by the heat flow to or from the system less the work (W) performed by the system on the environment:
- ,
which means that the energy of the system can change either via work or via heat flows across the boundary of the thermodynamic system. Internal energy is the sum of all forms of energy of a system, except those due to motion of the system as a whole. It is related to the molecular structure and to molecular motion and may be viewed as the sum of kinetic and potential energies of the molecules.
The transfer of heat to an ideal gas at constant pressure increases the internal energy and performs boundary work, i.e. allows a control volume of gas to become larger or smaller, provided the volume is not constrained. Returning to the first law equation and separating the work term into two types, boundary work and other, e.g., shaft work performed by a compressor fan, yields the following:
This combined quantity is enthalpy, , one of the thermodynamic potentials. Both enthalpy, , and internal energy, are state functions. State functions return to their initial values upon completion of each cycle in cyclic processes such as that of a heat engine. In contrast, neither nor are properties of a system and need not sum to zero over the steps of a cycle. The infinitesimal expression for heat, , forms an inexact differential for processes involving work. However, for processes involving no change in volume, applied magnetic field, or other external parameters, forms an exact differential. Likewise, for adiabatic processes (no heat transfer), the expression for work forms an exact differential, but for processes involving transfer of heat it forms an inexact differential.
Enthalpy and internal energy changes
Ideal gas
For a simple compressible system such as an ideal gas inside a piston, the changes in enthalpy and internal energy can be related to the heat capacity at constant pressure and volume, respectively. Constrained to have constant volume, the heat, , required to change its temperature from an initial temperature, T0, to a final temperature, Tf is given by:
Removing the volume constraint and allowing the system to expand or contract at constant pressure:
Incompressible substances
For incompressible substances, such as solids and liquids, the distinction between the two types of heat capacity (i.e. which is based on constant pressure and which is based on constant volume) disappears, as no work is performed.
Specific heat
Specific heat is defined as the amount of energy that has to be transferred to or from one unit of mass or mole of a substance to change its temperature by one degree. Specific heat is a property, which means that it depends on the substance under consideration and its state as specified by its properties. Fuels, when burned, are converted to molecules with a lower internal energy. The change in energy is heat. Upon changing from one phase to another, a pure substance releases or absorbs heat without its temperature changing. The amount of heat transfer during a phase change is known as latent heat and depends primarily on the substance and its state.
The specific heats of monatomic gases (e.g., helium) are nearly constant with temperature. Diatomic gases such as hydrogen display some temperature dependence, and triatomic gases (e.g., carbon dioxide) still more.
In liquids at sufficiently low temperatures, quantum effects become significant. An example is the behavior of bosons such as helium-4. For such substances, the behavior of heat capacity with temperature is discontinuous at the Bose-Einstein condensation point.
The quantum behavior of solids is adequately characterized by the Debye model. At temperatures well below the characteristic Debye temperature of a solid lattice, its specific heat will be proportional to the cube of absolute temperature. For low-temperature metals, a second term is needed to account for the behavior of the conduction electrons, an example of Fermi-Dirac statistics.
Calculating heat capacity from molar and specific heat capacity
The molar and specific heat capacities are dependent upon the internal degrees of freedom of the system and not on any bulk properties such as volume and number of molecules.
In contrast, heat capacity itself is an extensive quantity and as such is dependent on the number of molecules in the system. It can be represented as the product of mass, , and specific heat capacity, according to:
or is dependent on the number of moles and the molar heat capacity, according to:
Entropy
In 1856, German physicist Rudolf Clausius defined the second fundamental theorem (the second law of thermodynamics) in the mechanical theory of heat (thermodynamics): "if two transformations which, without necessitating any other permanent change, can mutually replace one another, be called equivalent, then the generations of the quantity of heat Q from work at the temperature T, has the equivalence-value:"[5][6]
In 1865, he came to define this ratio as entropy symbolized by S, such that, for a closed, stationary system:
and thus, by reduction, quantities of heat δQ (an inexact differential) are defined as quantities of TdS (an exact differential):
In other words, the entropy function S facilitates the quantification and measurement of heat flow through a thermodynamic boundary.
Heat transfer in engineering

The discipline of heat transfer, typically considered an aspect of mechanical engineering and chemical engineering, deals with specific applied methods by which heat transfer occurs. Note that although the definition of heat implicitly means the transfer of energy, the term heat transfer has acquired this traditional usage in engineering and other contexts. The understanding of heat transfer is crucial for the design and operation of numerous devices and processes.
Heat transfer may occur by the mechanisms of conduction, radiation, and mass transfer. In engineering, the term convective heat transfer is used to describe the combined effects of conduction and fluid flow and is often regarded as an additional mechanism of heat transfer. Although separate physical laws have been discovered to describe the behavior of each of these methods, real systems may exhibit a complicated combination. Various mathematical methods have been developed to solve or approximate the results of heat transfer in systems.
Semantic misconceptions
There is some debate in the scientific community regarding exactly how the term heat should be used.[7] In current scientific usage, the language surrounding the term can be conflicting and even misleading. One study showed that several popular textbooks used language that implied several meanings of the term, that heat is the process of transferring energy, that it is the transferred energy, i.e. as if it were a substance, and that is an entity contained within a system, among other similar descriptions. The study determined it was not uncommon for a combination of these representations to appear within the same text.[8] They found the predominant use among physicists to be that if it were a substance.
In a 2004 lecture, Friedrich Herrmann mentioned that the confusion may result from the modern practice of defining heat in terms energy, which is at odds both with the historic scientific definitions and with the modern lay concept of heat. He argues that the quantity heat as introduced by Joseph Black in the 18th century, and as used extensively by Sadi Carnot, was in fact what is today known as entropy-- something possessed by a substance in amounts related to that substance's temperature and mass, which exits one substance and enters another in the presence of a temperature gradient and can be created in many ways but never destroyed. He further argues that the layperson's concept of heat is also essentially this entropy concept, and so in re-defining heat to refer to an energy concept, modern science creates an unnecessarily awkward and confusing presentation of thermal physics. [9]
See also
References
- ^ a b
F. Reif (2000). Fundamentals of Statistical and Thermal Physics. Singapore: McGraw-Hll, Inc. p. 67. ISBN 0-07-Y85615-X.
{{cite book}}: Check|isbn=value: invalid character (help) - ^ Discourse on Heat and Work - Department of Physics and Astronomy, Georgia State University: Hyperphysics (online)
- ^
Schroeder, Daniel V. (2000). An introduction to thermal physics. San Francisco, California: Addison-Wesley. p. 18. ISBN 0-321-27779-1.
Heat is defined as any spontaneous flow of energy from one object to another, caused by a difference in temperature between the objects.
- ^ Smith, J.M., Van Ness, H.C., Abbot, M.M. (2005). Introduction to Chemical Engineering Thermodynamics. McGraw-Hill. ISBN 0073104450.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Published in Poggendoff’s Annalen, Dec. 1854, vol. xciii. p. 481; translated in the Journal de Mathematiques, vol. xx. Paris, 1855, and in the Philosophical Magazine, August 1856, s. 4. vol. xii, p. 81
- ^ Clausius, R. (1865). The Mechanical Theory of Heat] –with its Applications to the Steam Engine and to Physical Properties of Bodies. London: John van Voorst, 1 Paternoster Row. MDCCCLXVII.
- ^ "A review of selected literature on students' misconceptions of heat" (PDF). Boğaziçi University Journal of Education. 20 (1): 25–41. 2003.
- ^ Brookes, D.; Horton, G.; Van Heuvelen, A.; Etkina, E. (2005). "Concerning Scientific Discourse about Heat" (PDF). 2004 Physics Education Research Conference. 790. AIP Conference Proceedings: 149–152. doi:10.1063/1.2084723.
- ^ Herrmann, Friedrich (2004). "Entropy from the Beginning". In E. Mechlová (ed.). GIREP Conference 2004 Proceedings: Teaching and Learning Physics in new Contexts. University of Ostrava. pp. 35–40. ISBN 80-7042-378-1.
{{cite book}}:|access-date=requires|url=(help); Check date values in:|accessdate=(help); Cite has empty unknown parameter:|month=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)
External links
- Heat - article at Thermal-FluidsPedia.
- Plasma heat at 2 gigakelvins - Article about extremely high temperature generated by scientists (Foxnews.com)
- Correlations for Convective Heat Transfer - ChE Online Resources
- An Introduction to the Quantitative Definition and Analysis of Heat written for High School Students





















