Heat transfer: Difference between revisions
←Replaced content with 'Radiation is the heat transfer through the sun or a light bulb ''''''Bold text'''''''''Bold text'''''''''Bold text''''''''''''' Tag: blanking |
IronGargoyle (talk | contribs) m Reverted edits by 2601:2:5000:C21:2507:2344:B528:1334 (talk) to last revision by Brian the Editor (HG) |
||
| Line 1: | Line 1: | ||
[[File:Heat diffusion.png|thumb|Heat diffusion]] |
|||
Radiation is the heat transfer through the sun or a light bulb |
|||
'''Heat transfer''' is a discipline of [[thermal engineering]] that concerns the generation, use, conversion, and exchange of [[thermal energy]] and [[heat]] between physical systems. As such, heat transfer is involved in almost every sector of the economy.<ref>Taylor, R.A., ''Socioeconomic impacts of heat transfer research'', International Communications in Heat and Mass Transfer |
|||
''''''Bold text'''''''''Bold text'''''''''Bold text'''''''''''' |
|||
Volume 39, Issue 10, December 2012, Pages 1467–1473, http://www.sciencedirect.com/science/article/pii/S0735193312002199</ref> Heat transfer is classified into various mechanisms, such as [[thermal conduction]], [[convective heat transfer|thermal convection]], [[thermal radiation]], and transfer of energy by [[phase changes]]. Engineers also consider the transfer of mass of differing chemical species, either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system. |
|||
Heat conduction, also called diffusion, is the direct microscopic exchange of kinetic energy of particles through the boundary between two systems. When an object is at a different [[temperature]] from another body or its surroundings, [[heat]] flows so that the body and the surroundings reach the same temperature, at which point they are in [[thermal equilibrium]]. Such spontaneous heat transfer always occurs from a region of high temperature to another region of lower temperature, as described by the [[second law of thermodynamics]]. |
|||
Heat convection occurs when bulk flow of a fluid (gas or liquid) carries heat along with the flow of matter in the fluid. The flow of fluid may be forced by external processes, or sometimes (in gravitational fields) by buoyancy forces caused when thermal energy expands the fluid (for example in a fire plume), thus influencing its own transfer. The latter process is often called "natural convection". All convective processes also move heat partly by diffusion, as well. Another form of convection is forced convection. In this case the fluid is forced to flow by use of a pump, fan or other mechanical means. |
|||
Thermal radiation occurs through a [[vacuum]] or any [[transparency (optics)|transparent]] [[Optical medium|medium]] ([[solid]] or [[fluid]]). It is the transfer of energy by means of [[photons]] in [[electromagnetic waves]] governed by the same laws.<ref name=Geankoplis>{{cite book|last=Geankoplis|first=Christie John|title=Transport processes and separation process principles : (includes unit operations)|year=2003|publisher=Prentice Hall Professional Technical Reference|location=Upper Saddle River, NJ|isbn=0-13-101367-X|edition=4th ed.}}</ref> |
|||
[[File:Linear Heat flow.svg|thumb|Linear heat flow]] |
|||
==Overview== |
|||
[[Heat]] is defined in physics as the transfer of thermal energy across a well-defined boundary around a [[thermodynamic system]]. It is a characteristic of a process and is never ''contained'' in matter. In engineering contexts, however, the term ''heat transfer'' has acquired a specific usage, despite its literal redundancy of the characterization of transfer. In these contexts, ''heat'' is taken as synonymous to thermal energy. This usage has its origin in the historical interpretation of heat as a fluid (''caloric'') that can be transferred by various causes,<ref name=lienhard>{{cite book |
|||
|title = A Heat Transfer Textbook |
|||
| last = Lienhard |
|||
| first = John H.,V |
|||
| last2 = Lienhard |
|||
| first2 = John H., V |
|||
| edition = 3rd |
|||
| publisher = Phlogiston Press |
|||
| location = Cambridge, Massachusetts |
|||
| year = 2008 |
|||
| isbn = 978-0-9713835-3-1 |
|||
| oclc = 230956959 |
|||
}}</ref> and that is also common in the language of laymen and everyday life. |
|||
Fundamental methods of heat transfer in engineering include conduction, convection, and radiation. Physical laws describe the behavior and characteristics of each of these methods. Real systems often exhibit a complicated combination of them. Heat transfer methods are used in numerous disciplines, such as [[automotive engineering]], [[thermal management of electronic devices and systems]], [[HVAC|climate control]], [[thermal insulation|insulation]], [[process (engineering)|materials processing]], and [[power station]] engineering. |
|||
Various mathematical methods have been developed to solve or approximate the results of heat transfer in systems. Heat transfer is a [[process function]] (or path function), as opposed to [[functions of state]]; therefore, the amount of heat transferred in a [[thermodynamic process]] that changes the [[thermodynamic state|state]] of a [[thermodynamic system|system]] depends on how that process occurs, not only the net difference between the initial and final states of the process. [[Heat flux]] is a quantitative, vectorial representation of the heat flow through a surface.<ref name=NJIT>{{cite web|last=New Jersey Institute of Technology|first=Chemical Engineering Dept|title=B.S. Chemical Engineering|url=http://catalog.njit.edu/undergraduate/programs/chemicalengineering.php|publisher=NJIT|accessdate=9 April 2011}}</ref> |
|||
Heat transfer is typically studied as part of a general [[chemical engineering]] or [[mechanical engineering]] curriculum. Typically, [[thermodynamics]] is a [[wikt:prerequisite|prerequisite]] for heat transfer courses, as the laws of thermodynamics are essential to the mechanism of heat transfer.<ref name="NJIT"/> Other courses related to heat transfer include [[energy transformation]], [[thermal fluids]], and [[mass transfer]]. |
|||
The [[Transport phenomena|transport]] equations for thermal energy ([[Thermal conduction#Fourier's law|Fourier's law]]), mechanical momentum ([[Newtonian fluid|Newton's law for fluids]]), and mass transfer ([[Fick's laws of diffusion]]) are similar,<ref>{{cite book |
|||
|title=Fundamentals of momentum, heat, and mass transfer |
|||
|edition=2 |
|||
|first1=James R. |
|||
|last1=Welty |
|||
|first2=Charles E. |
|||
|last2=Wicks |
|||
|first3=Robert Elliott |
|||
|last3=Wilson |
|||
|publisher=Wiley |
|||
|location = New York |
|||
|isbn = 978-0-471-93354-0 |
|||
|oclc = 2213384 |
|||
|year=1976 |
|||
|url=http://books.google.be/books?cd=3&hl=en&id=hZxRAAAAMAAJ |
|||
}}</ref><ref name=Faghri>{{cite book |
|||
|title=Advanced Heat and Mass Transfer |
|||
|first1=Amir |
|||
|last1=Faghri |
|||
|first2=Yuwen |
|||
|last2=Zhang |
|||
|first3=John |
|||
|last3=Howell |
|||
|publisher=Global Digital Press |
|||
|location = Columbia, MO |
|||
|isbn = 978-0-9842760-0-4 |
|||
|year=2010 |
|||
}}</ref> |
|||
and analogies among these three transport processes have been developed to facilitate prediction of conversion from any one to the others.<ref name=Faghri/> |
|||
==Mechanisms== |
|||
The fundamental modes of heat transfer are: |
|||
;Conduction or diffusion |
|||
: The transfer of energy between objects that are in physical contact. |
|||
;Convection |
|||
: The transfer of energy between an object and its environment, due to fluid motion. |
|||
;Advection |
|||
: The transfer of energy from one location to another as a side effect of physically moving an object containing that energy. |
|||
;Radiation |
|||
: The transfer of energy to or from a body by means of the emission or absorption of electromagnetic radiation. |
|||
===Conduction=== |
|||
{{main|Thermal conduction}} |
|||
On a microscopic scale, heat conduction occurs as hot, rapidly moving or vibrating atoms and molecules interact with neighboring atoms and molecules, transferring some of their energy (heat) to these neighboring particles. In other words, heat is transferred by conduction when adjacent atoms vibrate against one another, or as electrons move from one atom to another. Conduction is the most significant means of heat transfer within a solid or between solid objects in [[thermal contact]]. Fluids—especially gases—are less conductive. [[Thermal contact conductance]] is the study of heat conduction between solid bodies in contact.<ref name=Abbott>{{cite book|last=Abbott|first=J.M. Smith, H.C. Van Ness, M.M.|title=Introduction to chemical engineering thermodynamics|year=2005|publisher=McGraw-Hill|location=Boston ; Montreal|isbn=0-07-310445-0|edition=7th ed.}}</ref> |
|||
''Steady state conduction'' (see [[Fourier's law]]) is a form of conduction that happens when the temperature difference driving the conduction is constant, so that after an equilibration time, the spatial distribution of temperatures in the conducting object does not change any further.<ref>[https://www.thermalfluidscentral.org/encyclopedia/index.php/Heat_Conduction "Thermal-FluidsPedia | Heat conduction"].</ref> In steady state conduction, the amount of heat entering a section is equal to amount of heat coming out.<ref name="Abbott"/> |
|||
''Transient conduction'' (see [[Heat equation]]) occurs when the temperature within an object changes as a function of time. Analysis of transient systems is more complex and often calls for the application of approximation theories or numerical analysis by computer.<ref name="Abbott"/> |
|||
{{Unicode|}} |
|||
===Convection=== |
|||
[[Convective heat transfer]], or convection, is the transfer of heat from one place to another by the movement of [[fluids]], a process that is essentially the transfer of heat via [[mass transfer]]. Bulk motion of fluid enhances heat transfer in many physical situations, such as (for example) between a solid surface and the fluid.<ref>{{cite book |
|||
| url = http://books.google.be/books?id=nrbfpSZTwskC&dq=9780072458930&hl=en&ei=1q7YTNKMJ8OqlAe25_CSCQ&sa=X&oi=book_result&ct=result&resnum=1&ved=0CCkQ6AEwAA |
|||
| title = Heat Transfer: a practical approach |
|||
| last = Çengel |
|||
| first = Yunus |
|||
| edition = 2nd |
|||
| publisher = McGraw-Hill |
|||
| location = Boston |
|||
| year = 2003 |
|||
| isbn = 978-0-07-245893-0 |
|||
| series = McGraw-Hill series in mechanical engineering. |
|||
| accessdate = 2009-04-20 |
|||
| oclc = 300472921 |
|||
}}</ref> |
|||
Convection is usually the dominant form of heat transfer in liquids and gases. Although sometimes discussed as a third method of heat transfer, convection is usually used to describe the combined effects of heat conduction within the fluid (diffusion) and heat transference by bulk fluid flow streaming.<ref>[https://www.thermalfluidscentral.org/encyclopedia/index.php/Convective_Heat_Transfer "Thermal-FluidsPedia | Convective heat transfer"]</ref> The process of transport by fluid streaming is known as [[advection]], but pure advection is a term that is generally associated only with mass transport in fluids, such as advection of pebbles in a river. In the case of heat transfer in fluids, where transport by advection in a fluid is always also accompanied by transport via heat diffusion (also known as heat conduction) the process of heat convection is understood to refer to the sum of heat transport by advection and diffusion/conduction. |
|||
Free, or natural, convection occurs when bulk fluid motions (steams and currents) are caused by buoyancy forces that result from density variations due to variations of temperature in the fluid. ''Forced'' convection is a term used when the streams and currents in the fluid are induced by external means—such as fans, stirrers, and pumps—creating an artificially induced convection current.<ref>{{cite web |
|||
| url = http://www.engineersedge.com/heat_transfer/convection.htm |
|||
| title = Convection — Heat Transfer |
|||
| work = Engineers Edge |
|||
| publisher = Engineers Edge |
|||
| accessdate = 2009-04-20 |
|||
}}</ref> |
|||
Convective heating or cooling in some circumstances may be described by [[Convective heat transfer#Newton's law of cooling|Newton's law of cooling]]: "The rate of heat loss of a body is proportional to the difference in temperatures between the body and its surroundings." However, by definition, the validity of Newton's law of cooling requires that the rate of heat loss from convection be a linear function of ("proportional to") the temperature difference that drives heat transfer, and in convective cooling this is sometimes not the case. In general, convection is not linearly dependent on temperature gradients, and in some cases is strongly nonlinear. In these cases, Newton's law does not apply. |
|||
<br>See also: [[Nusselt number]] |
|||
===Radiation=== |
|||
[[File:Hot metalwork.jpg|thumb|left|Red-hot iron object, transferring heat to the surrounding environment primarily through thermal radiation]] |
|||
[[Thermal radiation]] is energy emitted by matter as [[electromagnetic waves]], due to the pool of [[thermal energy]] in all matter with a temperature above [[absolute zero]]. Thermal radiation propagates without the presence of matter through the [[vacuum]] of space.<ref>[https://www.thermalfluidscentral.org/encyclopedia/index.php/Radiation "Thermal-FluidsPedia | Radiation"]</ref> |
|||
Thermal radiation is a direct result of the random movements of atoms and molecules in matter. Since these atoms and molecules are composed of charged particles ([[proton]]s and [[electron]]s), their movement results in the emission of [[electromagnetic radiation]], which carries energy away from the surface. |
|||
Radiation from the sun, or solar radiation, can be harvested for heat and power.<ref>Mojiri, A., ''[http://www.sciencedirect.com/science/article/pii/S1364032113005662 Spectral beam splitting for efficient conversion of solar energy—A review]'', Renewable and Sustainable Energy Reviews |
|||
Volume 28, December 2013, Pages 654–663</ref> Unlike conductive and convective forms of heat transfer, thermal radiation can be concentrated in a small spot by using reflecting mirrors, which is exploited in [[concentrating solar power]] generation.<ref>Taylor, R.A., ''[http://digitalcommons.lmu.edu/cgi/viewcontent.cgi?article=1019&context=mech_fac Applicability of Nanofluids in High Flux Solar Collectors]'' JOURNAL OF RENEWABLE AND SUSTAINABLE ENERGY 3, 023104, 2011</ref> For example, the sunlight reflected from mirrors heats the [[PS10 solar power tower]] and during the day it can heat water to {{convert|285|°C|°F}}.{{Citation needed|date=March 2011}} |
|||
{{clear}} |
|||
===Advection=== |
|||
By transferring matter, energy—including thermal energy—is moved by the physical transfer of a hot or cold object from one place to another.<ref>[https://www.thermalfluidscentral.org/encyclopedia/index.php/Mass_Transfer "Thermal-FluidsPedia | Mass transfer"]</ref> This can be as simple as placing hot water in a bottle and heating a bed, or the movement of an iceberg in changing ocean currents. A practical example is [[thermal hydraulics]].{{Citation needed|date=March 2011}} |
|||
This can be described by the formula |
|||
:<math>Q = v \cdot \rho \cdot c_p \cdot \Delta T</math> |
|||
where |
|||
Q is heat flux (W/m²), |
|||
ρ is density (kg/m³), |
|||
c_p is heat capacity at constant pressure (J/(kg*K)), |
|||
ΔT is the change in temperature (K), v is velocity (m/s). |
|||
===Convection vs. conduction=== |
|||
In a body of fluid that is heated from underneath its container, conduction and convection can be considered to compete for dominance. If heat conduction is too great, fluid moving down by convection is heated by conduction so fast that its downward movement will be stopped due to its [[buoyancy]], while fluid moving up by convection is cooled by conduction so fast that its driving buoyancy will diminish. On the other hand, if heat conduction is very low, a large temperature gradient may be formed and convection might be very strong. |
|||
The [[Rayleigh number]] (<math>Ra </math>) is a measure determining the relative strength of conduction and convection.{{Citation needed|date=March 2011}} |
|||
:<math> Ra = \frac{g \Delta \rho L^3} {\mu \alpha} = \frac{g \beta \Delta T L^3} {\nu \alpha}</math> |
|||
where |
|||
*''g'' is acceleration due to gravity, |
|||
*ρ is the density with <math>\Delta \rho</math> being the density difference between the lower and upper ends, |
|||
*μ is the [[dynamic viscosity]], |
|||
*α is the [[Thermal diffusivity]], |
|||
*β is the volume [[thermal expansivity]] (sometimes denoted ''α'' elsewhere), |
|||
*''T'' is the temperature, |
|||
*ν is the [[kinematic viscosity]], and |
|||
*''L'' is characteristic length. |
|||
The Rayleigh number can be understood as the ratio between the rate of heat transfer by convection to the rate of heat transfer by conduction; or, equivalently, the ratio between the corresponding timescales (i.e. conduction timescale divided by convection timescale), up to a numerical factor. This can be seen as follows, where all calculations are up to numerical factors depending on the geometry of the system. |
|||
The buoyancy force driving the convection is roughly <math>g \Delta \rho L^3</math>, so the corresponding pressure is roughly <math>g \Delta \rho L </math>. In [[steady state]], this is canceled by the shear stress due to viscosity, and therefore roughly equals <math>\mu V/L = \mu /T_{conv} </math>, where ''V'' is the typical fluid velocity due to convection and <math>T_{conv}</math> the order of its timescale.{{Citation needed|date=March 2011}} The conduction timescale, on the other hand, is of the order of <math>T_{cond} = L^2/ \alpha</math>. |
|||
Convection occurs when the Rayleigh number is above 1,000–2,000. |
|||
==Phase changes== |
|||
{{See also|Latent heat of fusion}} |
|||
Transfer of heat through a [[phase transition]] in the medium—such as water-to-ice, water-to-steam, steam-to-water, or ice-to-water—involves significant energy and is exploited in many ways: [[steam engine]]s, [[refrigerator]]s, etc.<ref>[https://www.thermalfluidscentral.org/encyclopedia/index.php/Multiphase_systems "Thermal-FluidsPedia | Multiphase systems"]</ref> For example, the [[Mason equation]] is an approximate analytical expression for the growth of a water droplet based on the effects of heat transport on [[evaporation]] and [[condensation]]. |
|||
===Boiling=== |
|||
Heat transfer in [[boiling]] fluids is complex, but of considerable technical importance. |
|||
At low driving temperatures, no boiling occurs and the heat transfer rate is controlled by the usual single-phase mechanisms. As the surface temperature is increased, local boiling occurs and vapor bubbles nucleate, grow into the surrounding cooler fluid, and collapse. This is ''sub-cooled nucleate boiling'', and is a very efficient heat transfer mechanism. At high bubble generation rates, the bubbles begin to interfere and the heat flux no longer increases rapidly with surface temperature (this is the [[Nucleate boiling#Departure from nucleate boiling|departure from nucleate boiling]], or DNB). At higher temperatures still, a maximum in the heat flux is reached (the [[critical heat flux]], or CHF). The regime of falling heat transfer that follows is not easy to study, but is believed to be characterized by alternate periods of nucleate and film boiling. |
|||
Nucleate boiling slows the heat transfer due to gas bubbles on the heater's surface; as mentioned, gas-phase thermal conductivity is much lower than liquid-phase thermal conductivity, so the outcome is a kind of "gas thermal barrier".{{Citation needed|date=March 2011}} |
|||
At higher temperatures still, the hydrodynamically-quieter regime of film boiling is reached. Heat fluxes across the stable vapor layers are low, but rise slowly with temperature. Any contact between fluid and the surface that may be seen probably leads to the extremely rapid nucleation of a fresh vapor layer ("spontaneous [[nucleation]]").{{Citation needed|date=March 2011}} |
|||
===Condensation=== |
|||
Condensation occurs when a vapor is cooled and changes its phase to a liquid. Condensation heat transfer, like boiling, is of great significance in industry.{{citation needed|date=November 2010}} During condensation, the [[latent heat of vaporization]] must be released. The amount of the heat is the same as that absorbed during vaporization at the same fluid pressure.{{Citation needed|date=March 2011}} |
|||
There are several types of condensation: |
|||
* Homogeneous condensation, as during a formation of fog. |
|||
* Condensation in direct contact with subcooled liquid. |
|||
* Condensation on direct contact with a cooling wall of a heat exchanger: This is the most common mode used in industry: |
|||
** Filmwise condensation is when a liquid film is formed on the subcooled surface, and usually occurs when the liquid wets the surface. |
|||
** Dropwise condensation is when liquid drops are formed on the subcooled surface, and usually occurs when the liquid does not wet the surface. |
|||
:Dropwise condensation is difficult to sustain reliably; therefore, industrial equipment is normally designed to operate in filmwise condensation mode. |
|||
==Modeling approaches== |
|||
Complex heat transfer phenomena can be modeled in different ways. |
|||
===Heat equation=== |
|||
The [[heat equation]] is an important [[partial differential equation]] that describes the distribution of heat (or variation in temperature) in a given region over time. In some cases, exact solutions of the equation are available; in other cases the equation must be solved numerically using [[Computational fluid dynamics|computational methods]]. For example, simplified [[climate model]]s may use Newtonian cooling, instead of a full (and computationally expensive) radiation code, to maintain atmospheric temperatures.{{Citation needed|date=March 2011}} |
|||
===Lumped system analysis=== |
|||
System analysis by the [[lumped capacitance model]] is a common approximation in transient conduction that may be used whenever heat conduction within an object is much faster than heat conduction across the boundary of the object. |
|||
This is a method of approximation that reduces one aspect of the transient conduction system—that within the object—to an equivalent steady state system. That is, the method assumes that the temperature within the object is completely uniform, although its value may be changing in time. |
|||
In this method, the ratio of the conductive heat resistance within the object to the convective heat transfer resistance across the object's boundary, known as the ''[[Biot number]]'', is calculated. For small Biot numbers, the approximation of ''spatially uniform temperature within the object'' can be used: it can be presumed that heat transferred into the object has time to uniformly distribute itself, due to the lower resistance to doing so, as compared with the resistance to heat entering the object.{{Citation needed|date=March 2011}} |
|||
Lumped system analysis often reduces the complexity of the equations to one first-order linear differential equation, in which case heating and cooling are described by a simple exponential solution, often referred to as [[Newton's law of cooling]]. |
|||
==Applications and techniques== |
|||
Heat transfer has broad application to the functioning of numerous devices and systems. Heat-transfer principles may be used to preserve, increase, or decrease temperature in a wide variety of circumstances.{{Citation needed|date=March 2011}} |
|||
===Insulation and radiant barriers=== |
|||
[[File:Plasma sprayed ceramic coating applied onto a part of an automotive exhaust system copy.jpg|thumb|right|Car exhausts usually require some form of heat barrier, especially high performance exhausts where a ceramic coating is often applied]] |
|||
[[File:Nelson tulsa test.jpg|thumb|right|Heat exposure as part of a fire test for firestop products]] |
|||
[[Thermal insulation|Thermal insulators]] are materials specifically designed to reduce the flow of heat by limiting conduction, convection, or both. [[Radiant barrier]]s are materials that [[Reflection (physics)|reflect]] radiation, and therefore reduce the flow of heat from radiation sources. Good insulators are not necessarily good radiant barriers, and vice versa. Metal, for instance, is an excellent reflector and a poor insulator. |
|||
The effectiveness of an insulator is indicated by its '''[[R-value (insulation)|R-value]]''', or resistance value. The R-value of a material is the [[Multiplicative inverse|inverse]] of the conduction coefficient (''k'') multiplied by the thickness (''d'') of the insulator. In most of the world, R-values are measured in [[SI]] units: square-meter kelvins per watt (m²·K/W). In the United States, R-values are customarily given in units of British thermal units per hour per square-foot degrees Fahrenheit (Btu/h·ft²·°F).{{Citation needed|date=March 2011}} |
|||
<math>{R} = {d \over k}</math> |
|||
<math>{C} = {Q \over m \Delta T}</math> |
|||
Rigid fiberglass, a common insulation material, has an R-value of four per inch, while poured concrete, a poor insulator, has an R-value of 0.08 per inch.<ref>{{cite web |
|||
| url = http://coloradoenergy.org/procorner/stuff/r-values.htm |
|||
| title = R-Value Table |
|||
| work = ColoradoENERGY.org |
|||
| date = 2008-07-29 |
|||
| accessdate = 2010-11-08 |
|||
| publisher = R. L. Martin & Associates, Inc. |
|||
| last = Martin |
|||
| first = Randy L. |
|||
| location = Windsor, Colorado |
|||
}}</ref> |
|||
The [[tog (unit)|tog]] is a measure of [[thermal resistance]], commonly used in the textile industry, and often seen quoted on, for example, duvets and carpet underlay.{{citation needed|date=November 2010}} |
|||
The effectiveness of a radiant barrier is indicated by its '''reflectivity''', which is the fraction of radiation reflected. A material with a high reflectivity (at a given wavelength) has a low emissivity (at that same wavelength), and vice versa. At any specific wavelength, reflectivity = 1 - emissivity. An ideal radiant barrier would have a reflectivity of 1, and would therefore reflect 100 percent of incoming radiation. [[Vacuum flasks]], or Dewars, are [[silvered]] to approach this ideal. In the vacuum of space, satellites use [[multi-layer insulation]], which consists of many layers of aluminized (shiny) [[Mylar]] to greatly reduce radiation heat transfer and control satellite temperature.{{Citation needed|date=March 2011}} |
|||
====Critical insulation thickness==== |
|||
Low thermal conductivity (''k'') materials reduce heat fluxes. The smaller the ''k'' value, the larger the corresponding thermal resistance (''R'') value. Thermal conductivity is measured in [[watt]]s-per-meter per [[kelvin]] (W·m<sup>−1</sup>·K<sup>−1</sup>), represented as ''k''. As the thickness of insulating material increases, the thermal resistance—or [[R-value (insulation)|R-value]]—also increases. |
|||
For a cylinder, the convective thermal resistance is inversely proportional to the surface area and therefore the radius of the cylinder, while the [[Thermal conduction#Cylindrical shells|thermal resistance of a cylindrical shell]] (the insulation layer) depends on the ratio between outside and inside radius, not on the radius itself. Suppose for example that we double the outside radius of a cylinder by applying insulation. We have added a fixed amount of conductive resistance (equal to ln(2)/(2πkL)) but at the same time we have halved the value of the convective resistance. Because convective resistance tends to infinity when the radius approaches zero, at small enough radiuses the decrease in convective resistance will be larger than the added conductive resistance, resulting in lower total resistance. <br /> |
|||
This implies that a critical radius exists at which the heat transfer is maximum. Above this critical radius, added insulation decreases the heat transfer. For insulated cylinders, the critical radius is given by the equation <ref>Bergman, Lavine, Incropera and DeWitt, ''Introduction to Heat Transfer'' (sixth edition), Wiley, 2011.</ref> |
|||
:<math>{r_{critical}} = {k \over h}</math> |
|||
This equation shows that the critical radius depends only on the heat transfer coefficient and the thermal conductivity of the insulation. If the radius of the uninsulated cylinder is larger than the critical radius for insulation, the addition of any amount of insulation will decrease the heat transfer. |
|||
====Heat exchangers==== |
|||
{{Main|Heat exchanger}} |
|||
A [[heat exchanger]] is a tool built for efficient heat transfer from one fluid to another, whether the fluids are separated by a solid wall so that they never mix, or the fluids are in direct contact. Heat exchangers are widely used in [[refrigeration]], [[air conditioning]], [[space heating]], [[power generation]], and chemical processing. One common example of a heat exchanger is a car's radiator, in which the hot [[coolant|coolant fluid]] is cooled by the flow of air over the radiator's surface.{{Citation needed|date=March 2011}} |
|||
Common types of heat exchanger flows include parallel flow, counter flow, and cross flow. In parallel flow, both fluids move in the same direction while transferring heat; in counter flow, the fluids move in opposite directions; and in cross flow, the fluids move at [[right angle]]s to each other. Common constructions for heat exchanger include shell and tube, [[Heat exchanger#Double pipe heat exchanger|double pipe]], extruded finned pipe, spiral fin pipe, u-tube, and stacked plate.{{Elucidate|date=November 2010}} |
|||
When engineers calculate the theoretical heat transfer in a heat exchanger, they must contend with the fact that the driving temperature difference between the two fluids varies with position. To account for this in simple systems, the [[log mean temperature difference]] (LMTD) is often used as an "average" temperature. In more complex systems, direct knowledge of the LMTD is not available, and the [[NTU method|number of transfer units]] (NTU) method can be used instead.{{Citation needed|date=March 2011}} |
|||
===Heat dissipation=== |
|||
A [[heat sink]] is a component that transfers heat generated within a solid material to a fluid medium, such as air or a liquid. Examples of heat sinks are the heat exchangers used in refrigeration and air conditioning systems, and the radiator in a car (which is also a heat exchanger). Heat sinks also help to cool electronic and optoelectronic devices such as [[CPU]]s, higher-power lasers, and light-emitting diodes (LEDs). A heat sink uses its extended surfaces to increase the surface area in contact with the cooling fluid. |
|||
====Buildings==== |
|||
In cold climates, houses with their heating systems form dissipative systems, often resulting in a loss of energy (known colloquially as "Heat Bleed") that makes home interiors uncomfortably cool or cold. |
|||
For the comfort of the inhabitants, the interiors must be maintained out of thermal equilibrium with the external surroundings. In effect, these domestic residences are islands of warmth in a sea of cold, and the thermal gradient between the inside and outside is often quite steep. This can lead to problems such as condensation and uncomfortable [[air current]]s, which—if left unaddressed—can cause cosmetic or structural damage to the property. |
|||
Such issues can be prevented through the execution of an [[energy audit]], and the implementation of recommended corrective procedures (such as the installation of adequate insulation, the air sealing of structural leaks, and the addition of energy-efficient windows and doors.<ref>{{cite web|title=EnergySavers: Tips on Saving Money & Energy at Home|url=http://www.energysavers.gov/pdfs/energy_savers.pdf|publisher=U.S. Department of Energy|accessdate=March 2, 2012}}</ref> |
|||
[[Thermal transmittance]] is the rate of transfer of heat through a structure divided by the difference in temperature across the structure. It is expressed in watts per square meter per kelvin, or W/m²K. Well-insulated parts of a building have a low thermal transmittance, whereas poorly-insulated parts of a building have a high thermal transmittance. |
|||
A [[thermostat]] is a device capable of starting the heating system when the house's interior falls below a set temperature, and of stopping that same system when another (higher) set temperature has been achieved. Thus, the thermostat controls the flow of energy into the house, that energy eventually being dissipated to the exterior.{{Citation needed|date=March 2011}} |
|||
===Thermal energy storage=== |
|||
[[Thermal energy storage]] refers to technologies that [[Energy storage|store energy]] in a thermal reservoir (such as a packed bed<ref>{{cite journal|last=Kuznetsov|first=A. V.|title=A Perturbation Solution for a Nonthermal Equilibrium Fluid Flow Through a Three-Dimensional Sensible Heat Storage Packed Bed|journal=Journal of Heat Transfer|year=1996|volume=118|issue=2|pages=508|doi=10.1115/1.2825881}}</ref> ) for later use. They can be employed to balance energy demand between daytime and nighttime. The thermal reservoir may be maintained at a temperature above (hotter) or below (colder) than that of the ambient environment. Applications include later use in space heating, domestic or process hot water, or to generate electricity. Most practical active solar heating systems have storage for a few hours to a day's worth of heat collected in insulated hot water tanks, but this can be extended to storage between opposing seasons by using underground [[seasonal thermal energy storage]].<ref>{{cite web|url=http://www.icax.co.uk/Underground_Thermal_Energy_Storage.html |title=Underground Thermal Energy Storage |publisher=Icax.co.uk |date= |accessdate=2012-06-30}}</ref> |
|||
===Evaporative cooling=== |
|||
[[Evaporative cooling]] is a physical phenomenon in which evaporation of a liquid, typically into surrounding air, cools an object or a liquid in contact with it. Latent heat describes the amount of heat that is needed to evaporate the liquid; this heat comes from the liquid itself and the surrounding gas and surfaces. The greater the difference between the two temperatures, the greater the evaporative cooling effect. When the temperatures are the same, no net evaporation of water in air occurs; thus, there is no cooling effect. A simple example of natural evaporative cooling is perspiration, or sweat, which the body secretes in order to cool itself. An [[evaporative cooler]] is a device that cools air through the simple evaporation of water.{{Citation needed|date=March 2011}} |
|||
===Radiative cooling=== |
|||
[[Radiative cooling]] is the process by which a body loses heat by radiation. It is an important effect in the Earth's atmosphere. In the case of the Earth-atmosphere system, it refers to the process by which long-wave (infrared) radiation is emitted to balance the absorption of short-wave (visible) energy from the Sun. Convective transport of heat and evaporative transport of latent heat both remove heat from the surface and redistribute it in the atmosphere, making it available for radiative transport at higher altitudes.{{Citation needed|date=March 2011}} |
|||
===Laser cooling=== |
|||
[[Laser cooling]] refers to techniques in which atomic and molecular samples are cooled through the interaction with one or more laser light fields. The most common method of laser cooling is [[Doppler cooling]]. In Doppler cooling, the frequency of the laser light is tuned slightly below an [[Energy level|electronic transition]] in the atom. Thus, the atoms would absorb more photons if they moved towards the light source, due to the [[Doppler effect]]. If an excited atom then emits a photon spontaneously, it will be accelerated. The result of the absorption and emission process is to reduce the speed of the atom. Eventually the mean velocity, and therefore the kinetic energy of the atoms, will be reduced. Since the temperature of an ensemble of atoms is a measure of the random internal kinetic energy, this is equivalent to cooling the atoms. |
|||
[[Sympathetic cooling]] is a process in which particles of one type cool particles of another type. Typically, atomic ions that can be directly laser-cooled are used to cool nearby ions or atoms. This technique allows cooling of ions and atoms that cannot be laser cooled directly.{{Citation needed|date=March 2011}} |
|||
===Magnetic cooling=== |
|||
[[Magnetic evaporative cooling]] is a technique for lowering the temperature of a group of atoms. The process confines atoms using a magnetic field. Over time, individual atoms will become much more energetic than the others due to random collisions, and will escape—removing energy from the system and reducing the temperature of the remaining group. This process is similar to the familiar process by which standing water becomes water vapor.{{Citation needed|date=March 2011}} |
|||
===Heat transfer in the human body=== |
|||
The principles of heat transfer in engineering systems can be applied to the human body in order to determine how the body transfers heat. Heat is produced in the body by the continuous metabolism of nutrients which provides energy for the systems of the body.<ref>Hartman,Carl and Bibb, Lewis. "The Human body and its enemies: a textbook of physiology hygiene and sanitation", World Book Co., 1913, p.232.</ref> The human body must maintain a consistent internal temperature in order to maintain healthy bodily functions. Therefore, excess heat must be dissipated from the body to keep it from overheating. When a person engages in elevated levels of physical activity, the body requires additional fuel which increases the metabolic rate and the rate of heat production. The body must then use additional methods to remove the additional heat produced in order to keep the internal temperature at a healthy level. |
|||
[[Convective heat transfer|Heat transfer by convection]] is driven by the movement of fluids over the surface of the body. This convective fluid can be either a liquid or a gas. For heat transfer from the outer surface of the body, the convection mechanism is dependent on the surface area of the body, the velocity of the air, and the temperature gradient between the surface of the skin and the ambient air.<ref name="Cengel, Yunus A 2010">Cengel, Yunus A. and Ghajar, Afshin J. "Heat and Mass Transfer: Fundamentals and Applications." , McGraw-Hill, 4th Edition, 2010.</ref> The normal temperature of the body is approximately 37°C. Heat transfer occurs more readily when the temperature of the surroundings is significantly less than the normal body temperature. This concept explains why a person feels “cold” when not enough covering is worn when exposed to a cold environment. Clothing can be considered an insulator which provides thermal resistance to heat flow over the covered portion of the body.<ref>Tao, Xiaoming. "Smart fibres, fabrics, and clothing" , Woodhead Publishing, 2001</ref> This thermal resistance causes the temperature on the surface of the clothing to be less than the temperature on the surface of the skin. This smaller temperature gradient between the surface temperature and the ambient temperature will cause a lower rate of heat transfer than if the skin were not covered. |
|||
In order to ensure that one portion of the body is not significantly hotter than another portion, heat must be distributed evenly through the bodily tissues. Blood flowing through blood vessels acts as a convective fluid and helps to prevent any buildup of excess heat inside the tissues of the body. This flow of blood through the vessels can be modeled as pipe flow in an engineering system. The heat carried by the blood is determined by the temperature of the surrounding tissue, the diameter of the blood vessel, the [[Viscosity|thickness of the fluid]], velocity of the flow, and the heat transfer coefficient of the blood. The velocity, blood vessel diameter, and the fluid thickness can all be related with the [[Reynolds Number]], a dimensionless number used in fluid mechanics to characterize the flow of fluids. |
|||
[[Latent heat]] loss, also known as evaporative heat loss, accounts for a large fraction of heat loss from the body. When the core temperature of the body increases, the body triggers sweat glands in the skin to bring additional moisture to the surface of the skin. The liquid is then transformed into vapor which removes heat from the surface of the body.<ref>Wilmore, Jack H., Costill, David L., Kenney, Larry, "Physiology of sport and exercise", Human Kinetics, 2008, p.256.</ref> The rate of evaporation heat loss is directly related to the vapor pressure at the skin surface and the amount of moisture present on the skin.<ref name="Cengel, Yunus A 2010"/> Therefore, the maximum of heat transfer will occur when the skin is completely wet. The body continuously loses water by evaporation but the most significant amount of heat loss occurs during periods of increased physical activity. |
|||
===Other=== |
|||
A [[heat pipe]] is a passive device constructed in such a way that it acts as though it has extremely high thermal conductivity. Heat pipes use latent heat and capillary action to move heat, and can carry many times as much heat as a similar-sized copper rod. Originally invented for use in [[satellites]], they have applications in [[personal computer]]s. Another major use is for solar hot water panels. |
|||
A [[thermocouple]] is a junction between two different metals that produces a voltage related to a temperature difference. Thermocouples are a widely used type of temperature sensor for measurement and control, and can also be used to convert heat into electric power. |
|||
A [[thermopile]] is an electronic device that converts thermal energy into electrical energy. It is composed of thermocouples. Thermopiles do not measure the absolute temperature, but generate an output voltage proportional to a temperature difference. Thermopiles are widely used, e.g., they are the key component of [[infrared thermometer]]s, such as those used to measure body temperature via the ear.{{citation needed|date=November 2010}} |
|||
A [[thermoelectric cooler]] is a solid state electronic device that pumps (transfers) heat from one side of the device to the other when electrical current is passed through it. It is based on the [[Peltier effect]]. |
|||
A [[thermal diode]] or [[thermal rectifier]] is a device that preferentially passes heat in one direction: a "one-way valve" for heat.{{Citation needed|date=March 2011}} |
|||
==See also== |
|||
*[[Boiling]] |
|||
*[[Combined forced and natural convection]] |
|||
*[[Heat transfer physics]] |
|||
*[[Stefan–Boltzmann law]] |
|||
*[[Thermal contact conductance]] |
|||
*[[Thermal physics]] |
|||
*[[Thermal resistance in electronics]] |
|||
*[[Thermal science]] |
|||
==References== |
|||
{{Reflist|2}} |
|||
==Further reading== |
|||
*[https://www.thermalfluidscentral.org/e-journals/index.php/Heat_Mass_Transfer ''Frontiers in Heat and Mass Transfer''] |
|||
*[http://www.tandf.co.uk/journals/titles/01457632.asp ''Heat Transfer Engineering''] |
|||
*[http://www.tandf.co.uk/journals/titles/08916152.asp ''Experimental Heat Transfer''] |
|||
*[http://www.sciencedirect.com/science/journal/00179310 ''International Journal of Heat and Mass Transfer''] |
|||
*[http://scitation.aip.org/dbt/dbt.jsp?KEY=JHTRAO ''ASME Journal of Heat Transfer''] |
|||
*[http://www.tandf.co.uk/journals/titles/10407782.asp ''Numerical Heat Transfer Part A''] |
|||
*[http://www.tandf.co.uk/journals/titles/10407790.asp ''Numerical Heat Transfer Part B''] |
|||
*[http://www.tandf.co.uk/journals/titles/15567265.asp ''Nanoscale and Microscale Thermophysical Engineering''] |
|||
*[http://www.begellhouse.com/journals/4c8f5faa331b09ea.html ''Journal of Enhanced Heat Transfer''] |
|||
==External links== |
|||
*[https://www.thermalfluidscentral.org/encyclopedia/index.php/Main_Page Thermal-FluidsPedia] - An online thermal fluids encyclopedia. |
|||
*[http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/heatra.html Hyperphysics Article on Heat Transfer] - Overview |
|||
*[http://www.icax.co.uk/thermalbank.html Interseasonal Heat Transfer] - a practical example of how heat transfer is used to heat buildings without burning fossil fuels. |
|||
*[http://www.msm.cam.ac.uk/phase-trans/2007/HT/heat_transfer.html Aspects of Heat Transfer, Cambridge University] |
|||
*[https://www.thermalfluidscentral.org/ Thermal-Fluids Central] |
|||
*[http://energy.concord.org/energy2d/ Energy2D: Interactive Heat Transfer Simulations for Everyone] |
|||
*[http://anorkey.com/wp-content/uploads/2013/05/HMTSheet.pdf Heat and Mass Transport Equation Sheet] |
|||
{{Chemical engg}} |
|||
{{Use dmy dates|date=June 2011}} |
|||
{{DEFAULTSORT:Heat Transfer}} |
|||
[[Category:Mechanical engineering]] |
|||
[[Category:Chemical engineering]] |
|||
[[Category:Transport phenomena]] |
|||
[[Category:Heat transfer| ]] |
|||
[[Category:Heat conduction]] |
|||
Revision as of 22:30, 6 March 2014

Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy and heat between physical systems. As such, heat transfer is involved in almost every sector of the economy.[1] Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species, either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system.
Heat conduction, also called diffusion, is the direct microscopic exchange of kinetic energy of particles through the boundary between two systems. When an object is at a different temperature from another body or its surroundings, heat flows so that the body and the surroundings reach the same temperature, at which point they are in thermal equilibrium. Such spontaneous heat transfer always occurs from a region of high temperature to another region of lower temperature, as described by the second law of thermodynamics.
Heat convection occurs when bulk flow of a fluid (gas or liquid) carries heat along with the flow of matter in the fluid. The flow of fluid may be forced by external processes, or sometimes (in gravitational fields) by buoyancy forces caused when thermal energy expands the fluid (for example in a fire plume), thus influencing its own transfer. The latter process is often called "natural convection". All convective processes also move heat partly by diffusion, as well. Another form of convection is forced convection. In this case the fluid is forced to flow by use of a pump, fan or other mechanical means.
Thermal radiation occurs through a vacuum or any transparent medium (solid or fluid). It is the transfer of energy by means of photons in electromagnetic waves governed by the same laws.[2]
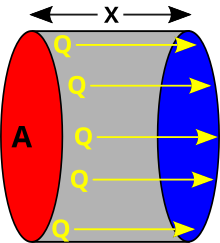
Overview
Heat is defined in physics as the transfer of thermal energy across a well-defined boundary around a thermodynamic system. It is a characteristic of a process and is never contained in matter. In engineering contexts, however, the term heat transfer has acquired a specific usage, despite its literal redundancy of the characterization of transfer. In these contexts, heat is taken as synonymous to thermal energy. This usage has its origin in the historical interpretation of heat as a fluid (caloric) that can be transferred by various causes,[3] and that is also common in the language of laymen and everyday life.
Fundamental methods of heat transfer in engineering include conduction, convection, and radiation. Physical laws describe the behavior and characteristics of each of these methods. Real systems often exhibit a complicated combination of them. Heat transfer methods are used in numerous disciplines, such as automotive engineering, thermal management of electronic devices and systems, climate control, insulation, materials processing, and power station engineering.
Various mathematical methods have been developed to solve or approximate the results of heat transfer in systems. Heat transfer is a process function (or path function), as opposed to functions of state; therefore, the amount of heat transferred in a thermodynamic process that changes the state of a system depends on how that process occurs, not only the net difference between the initial and final states of the process. Heat flux is a quantitative, vectorial representation of the heat flow through a surface.[4]
Heat transfer is typically studied as part of a general chemical engineering or mechanical engineering curriculum. Typically, thermodynamics is a prerequisite for heat transfer courses, as the laws of thermodynamics are essential to the mechanism of heat transfer.[4] Other courses related to heat transfer include energy transformation, thermal fluids, and mass transfer.
The transport equations for thermal energy (Fourier's law), mechanical momentum (Newton's law for fluids), and mass transfer (Fick's laws of diffusion) are similar,[5][6] and analogies among these three transport processes have been developed to facilitate prediction of conversion from any one to the others.[6]
Mechanisms
The fundamental modes of heat transfer are:
- Conduction or diffusion
- The transfer of energy between objects that are in physical contact.
- Convection
- The transfer of energy between an object and its environment, due to fluid motion.
- Advection
- The transfer of energy from one location to another as a side effect of physically moving an object containing that energy.
- Radiation
- The transfer of energy to or from a body by means of the emission or absorption of electromagnetic radiation.
Conduction
On a microscopic scale, heat conduction occurs as hot, rapidly moving or vibrating atoms and molecules interact with neighboring atoms and molecules, transferring some of their energy (heat) to these neighboring particles. In other words, heat is transferred by conduction when adjacent atoms vibrate against one another, or as electrons move from one atom to another. Conduction is the most significant means of heat transfer within a solid or between solid objects in thermal contact. Fluids—especially gases—are less conductive. Thermal contact conductance is the study of heat conduction between solid bodies in contact.[7]
Steady state conduction (see Fourier's law) is a form of conduction that happens when the temperature difference driving the conduction is constant, so that after an equilibration time, the spatial distribution of temperatures in the conducting object does not change any further.[8] In steady state conduction, the amount of heat entering a section is equal to amount of heat coming out.[7]
Transient conduction (see Heat equation) occurs when the temperature within an object changes as a function of time. Analysis of transient systems is more complex and often calls for the application of approximation theories or numerical analysis by computer.[7]
Convection
Convective heat transfer, or convection, is the transfer of heat from one place to another by the movement of fluids, a process that is essentially the transfer of heat via mass transfer. Bulk motion of fluid enhances heat transfer in many physical situations, such as (for example) between a solid surface and the fluid.[9] Convection is usually the dominant form of heat transfer in liquids and gases. Although sometimes discussed as a third method of heat transfer, convection is usually used to describe the combined effects of heat conduction within the fluid (diffusion) and heat transference by bulk fluid flow streaming.[10] The process of transport by fluid streaming is known as advection, but pure advection is a term that is generally associated only with mass transport in fluids, such as advection of pebbles in a river. In the case of heat transfer in fluids, where transport by advection in a fluid is always also accompanied by transport via heat diffusion (also known as heat conduction) the process of heat convection is understood to refer to the sum of heat transport by advection and diffusion/conduction.
Free, or natural, convection occurs when bulk fluid motions (steams and currents) are caused by buoyancy forces that result from density variations due to variations of temperature in the fluid. Forced convection is a term used when the streams and currents in the fluid are induced by external means—such as fans, stirrers, and pumps—creating an artificially induced convection current.[11]
Convective heating or cooling in some circumstances may be described by Newton's law of cooling: "The rate of heat loss of a body is proportional to the difference in temperatures between the body and its surroundings." However, by definition, the validity of Newton's law of cooling requires that the rate of heat loss from convection be a linear function of ("proportional to") the temperature difference that drives heat transfer, and in convective cooling this is sometimes not the case. In general, convection is not linearly dependent on temperature gradients, and in some cases is strongly nonlinear. In these cases, Newton's law does not apply.
See also: Nusselt number
Radiation

Thermal radiation is energy emitted by matter as electromagnetic waves, due to the pool of thermal energy in all matter with a temperature above absolute zero. Thermal radiation propagates without the presence of matter through the vacuum of space.[12]
Thermal radiation is a direct result of the random movements of atoms and molecules in matter. Since these atoms and molecules are composed of charged particles (protons and electrons), their movement results in the emission of electromagnetic radiation, which carries energy away from the surface.
Radiation from the sun, or solar radiation, can be harvested for heat and power.[13] Unlike conductive and convective forms of heat transfer, thermal radiation can be concentrated in a small spot by using reflecting mirrors, which is exploited in concentrating solar power generation.[14] For example, the sunlight reflected from mirrors heats the PS10 solar power tower and during the day it can heat water to 285 °C (545 °F).[citation needed]
Advection
By transferring matter, energy—including thermal energy—is moved by the physical transfer of a hot or cold object from one place to another.[15] This can be as simple as placing hot water in a bottle and heating a bed, or the movement of an iceberg in changing ocean currents. A practical example is thermal hydraulics.[citation needed] This can be described by the formula
where
Q is heat flux (W/m²), ρ is density (kg/m³), c_p is heat capacity at constant pressure (J/(kg*K)), ΔT is the change in temperature (K), v is velocity (m/s).
Convection vs. conduction
In a body of fluid that is heated from underneath its container, conduction and convection can be considered to compete for dominance. If heat conduction is too great, fluid moving down by convection is heated by conduction so fast that its downward movement will be stopped due to its buoyancy, while fluid moving up by convection is cooled by conduction so fast that its driving buoyancy will diminish. On the other hand, if heat conduction is very low, a large temperature gradient may be formed and convection might be very strong.
The Rayleigh number () is a measure determining the relative strength of conduction and convection.[citation needed]
where
- g is acceleration due to gravity,
- ρ is the density with being the density difference between the lower and upper ends,
- μ is the dynamic viscosity,
- α is the Thermal diffusivity,
- β is the volume thermal expansivity (sometimes denoted α elsewhere),
- T is the temperature,
- ν is the kinematic viscosity, and
- L is characteristic length.
The Rayleigh number can be understood as the ratio between the rate of heat transfer by convection to the rate of heat transfer by conduction; or, equivalently, the ratio between the corresponding timescales (i.e. conduction timescale divided by convection timescale), up to a numerical factor. This can be seen as follows, where all calculations are up to numerical factors depending on the geometry of the system.
The buoyancy force driving the convection is roughly , so the corresponding pressure is roughly . In steady state, this is canceled by the shear stress due to viscosity, and therefore roughly equals , where V is the typical fluid velocity due to convection and the order of its timescale.[citation needed] The conduction timescale, on the other hand, is of the order of .
Convection occurs when the Rayleigh number is above 1,000–2,000.
Phase changes
Transfer of heat through a phase transition in the medium—such as water-to-ice, water-to-steam, steam-to-water, or ice-to-water—involves significant energy and is exploited in many ways: steam engines, refrigerators, etc.[16] For example, the Mason equation is an approximate analytical expression for the growth of a water droplet based on the effects of heat transport on evaporation and condensation.
Boiling
Heat transfer in boiling fluids is complex, but of considerable technical importance.
At low driving temperatures, no boiling occurs and the heat transfer rate is controlled by the usual single-phase mechanisms. As the surface temperature is increased, local boiling occurs and vapor bubbles nucleate, grow into the surrounding cooler fluid, and collapse. This is sub-cooled nucleate boiling, and is a very efficient heat transfer mechanism. At high bubble generation rates, the bubbles begin to interfere and the heat flux no longer increases rapidly with surface temperature (this is the departure from nucleate boiling, or DNB). At higher temperatures still, a maximum in the heat flux is reached (the critical heat flux, or CHF). The regime of falling heat transfer that follows is not easy to study, but is believed to be characterized by alternate periods of nucleate and film boiling. Nucleate boiling slows the heat transfer due to gas bubbles on the heater's surface; as mentioned, gas-phase thermal conductivity is much lower than liquid-phase thermal conductivity, so the outcome is a kind of "gas thermal barrier".[citation needed]
At higher temperatures still, the hydrodynamically-quieter regime of film boiling is reached. Heat fluxes across the stable vapor layers are low, but rise slowly with temperature. Any contact between fluid and the surface that may be seen probably leads to the extremely rapid nucleation of a fresh vapor layer ("spontaneous nucleation").[citation needed]
Condensation
Condensation occurs when a vapor is cooled and changes its phase to a liquid. Condensation heat transfer, like boiling, is of great significance in industry.[citation needed] During condensation, the latent heat of vaporization must be released. The amount of the heat is the same as that absorbed during vaporization at the same fluid pressure.[citation needed]
There are several types of condensation:
- Homogeneous condensation, as during a formation of fog.
- Condensation in direct contact with subcooled liquid.
- Condensation on direct contact with a cooling wall of a heat exchanger: This is the most common mode used in industry:
- Filmwise condensation is when a liquid film is formed on the subcooled surface, and usually occurs when the liquid wets the surface.
- Dropwise condensation is when liquid drops are formed on the subcooled surface, and usually occurs when the liquid does not wet the surface.
- Dropwise condensation is difficult to sustain reliably; therefore, industrial equipment is normally designed to operate in filmwise condensation mode.
Modeling approaches
Complex heat transfer phenomena can be modeled in different ways.
Heat equation
The heat equation is an important partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time. In some cases, exact solutions of the equation are available; in other cases the equation must be solved numerically using computational methods. For example, simplified climate models may use Newtonian cooling, instead of a full (and computationally expensive) radiation code, to maintain atmospheric temperatures.[citation needed]
Lumped system analysis
System analysis by the lumped capacitance model is a common approximation in transient conduction that may be used whenever heat conduction within an object is much faster than heat conduction across the boundary of the object.
This is a method of approximation that reduces one aspect of the transient conduction system—that within the object—to an equivalent steady state system. That is, the method assumes that the temperature within the object is completely uniform, although its value may be changing in time.
In this method, the ratio of the conductive heat resistance within the object to the convective heat transfer resistance across the object's boundary, known as the Biot number, is calculated. For small Biot numbers, the approximation of spatially uniform temperature within the object can be used: it can be presumed that heat transferred into the object has time to uniformly distribute itself, due to the lower resistance to doing so, as compared with the resistance to heat entering the object.[citation needed]
Lumped system analysis often reduces the complexity of the equations to one first-order linear differential equation, in which case heating and cooling are described by a simple exponential solution, often referred to as Newton's law of cooling.
Applications and techniques
Heat transfer has broad application to the functioning of numerous devices and systems. Heat-transfer principles may be used to preserve, increase, or decrease temperature in a wide variety of circumstances.[citation needed]
Insulation and radiant barriers


Thermal insulators are materials specifically designed to reduce the flow of heat by limiting conduction, convection, or both. Radiant barriers are materials that reflect radiation, and therefore reduce the flow of heat from radiation sources. Good insulators are not necessarily good radiant barriers, and vice versa. Metal, for instance, is an excellent reflector and a poor insulator.
The effectiveness of an insulator is indicated by its R-value, or resistance value. The R-value of a material is the inverse of the conduction coefficient (k) multiplied by the thickness (d) of the insulator. In most of the world, R-values are measured in SI units: square-meter kelvins per watt (m²·K/W). In the United States, R-values are customarily given in units of British thermal units per hour per square-foot degrees Fahrenheit (Btu/h·ft²·°F).[citation needed]
Rigid fiberglass, a common insulation material, has an R-value of four per inch, while poured concrete, a poor insulator, has an R-value of 0.08 per inch.[17]
The tog is a measure of thermal resistance, commonly used in the textile industry, and often seen quoted on, for example, duvets and carpet underlay.[citation needed]
The effectiveness of a radiant barrier is indicated by its reflectivity, which is the fraction of radiation reflected. A material with a high reflectivity (at a given wavelength) has a low emissivity (at that same wavelength), and vice versa. At any specific wavelength, reflectivity = 1 - emissivity. An ideal radiant barrier would have a reflectivity of 1, and would therefore reflect 100 percent of incoming radiation. Vacuum flasks, or Dewars, are silvered to approach this ideal. In the vacuum of space, satellites use multi-layer insulation, which consists of many layers of aluminized (shiny) Mylar to greatly reduce radiation heat transfer and control satellite temperature.[citation needed]
Critical insulation thickness
Low thermal conductivity (k) materials reduce heat fluxes. The smaller the k value, the larger the corresponding thermal resistance (R) value. Thermal conductivity is measured in watts-per-meter per kelvin (W·m−1·K−1), represented as k. As the thickness of insulating material increases, the thermal resistance—or R-value—also increases.
For a cylinder, the convective thermal resistance is inversely proportional to the surface area and therefore the radius of the cylinder, while the thermal resistance of a cylindrical shell (the insulation layer) depends on the ratio between outside and inside radius, not on the radius itself. Suppose for example that we double the outside radius of a cylinder by applying insulation. We have added a fixed amount of conductive resistance (equal to ln(2)/(2πkL)) but at the same time we have halved the value of the convective resistance. Because convective resistance tends to infinity when the radius approaches zero, at small enough radiuses the decrease in convective resistance will be larger than the added conductive resistance, resulting in lower total resistance.
This implies that a critical radius exists at which the heat transfer is maximum. Above this critical radius, added insulation decreases the heat transfer. For insulated cylinders, the critical radius is given by the equation [18]
This equation shows that the critical radius depends only on the heat transfer coefficient and the thermal conductivity of the insulation. If the radius of the uninsulated cylinder is larger than the critical radius for insulation, the addition of any amount of insulation will decrease the heat transfer.
Heat exchangers
A heat exchanger is a tool built for efficient heat transfer from one fluid to another, whether the fluids are separated by a solid wall so that they never mix, or the fluids are in direct contact. Heat exchangers are widely used in refrigeration, air conditioning, space heating, power generation, and chemical processing. One common example of a heat exchanger is a car's radiator, in which the hot coolant fluid is cooled by the flow of air over the radiator's surface.[citation needed]
Common types of heat exchanger flows include parallel flow, counter flow, and cross flow. In parallel flow, both fluids move in the same direction while transferring heat; in counter flow, the fluids move in opposite directions; and in cross flow, the fluids move at right angles to each other. Common constructions for heat exchanger include shell and tube, double pipe, extruded finned pipe, spiral fin pipe, u-tube, and stacked plate.[further explanation needed]
When engineers calculate the theoretical heat transfer in a heat exchanger, they must contend with the fact that the driving temperature difference between the two fluids varies with position. To account for this in simple systems, the log mean temperature difference (LMTD) is often used as an "average" temperature. In more complex systems, direct knowledge of the LMTD is not available, and the number of transfer units (NTU) method can be used instead.[citation needed]
Heat dissipation
A heat sink is a component that transfers heat generated within a solid material to a fluid medium, such as air or a liquid. Examples of heat sinks are the heat exchangers used in refrigeration and air conditioning systems, and the radiator in a car (which is also a heat exchanger). Heat sinks also help to cool electronic and optoelectronic devices such as CPUs, higher-power lasers, and light-emitting diodes (LEDs). A heat sink uses its extended surfaces to increase the surface area in contact with the cooling fluid.
Buildings
In cold climates, houses with their heating systems form dissipative systems, often resulting in a loss of energy (known colloquially as "Heat Bleed") that makes home interiors uncomfortably cool or cold.
For the comfort of the inhabitants, the interiors must be maintained out of thermal equilibrium with the external surroundings. In effect, these domestic residences are islands of warmth in a sea of cold, and the thermal gradient between the inside and outside is often quite steep. This can lead to problems such as condensation and uncomfortable air currents, which—if left unaddressed—can cause cosmetic or structural damage to the property.
Such issues can be prevented through the execution of an energy audit, and the implementation of recommended corrective procedures (such as the installation of adequate insulation, the air sealing of structural leaks, and the addition of energy-efficient windows and doors.[19]
Thermal transmittance is the rate of transfer of heat through a structure divided by the difference in temperature across the structure. It is expressed in watts per square meter per kelvin, or W/m²K. Well-insulated parts of a building have a low thermal transmittance, whereas poorly-insulated parts of a building have a high thermal transmittance.
A thermostat is a device capable of starting the heating system when the house's interior falls below a set temperature, and of stopping that same system when another (higher) set temperature has been achieved. Thus, the thermostat controls the flow of energy into the house, that energy eventually being dissipated to the exterior.[citation needed]
Thermal energy storage
Thermal energy storage refers to technologies that store energy in a thermal reservoir (such as a packed bed[20] ) for later use. They can be employed to balance energy demand between daytime and nighttime. The thermal reservoir may be maintained at a temperature above (hotter) or below (colder) than that of the ambient environment. Applications include later use in space heating, domestic or process hot water, or to generate electricity. Most practical active solar heating systems have storage for a few hours to a day's worth of heat collected in insulated hot water tanks, but this can be extended to storage between opposing seasons by using underground seasonal thermal energy storage.[21]
Evaporative cooling
Evaporative cooling is a physical phenomenon in which evaporation of a liquid, typically into surrounding air, cools an object or a liquid in contact with it. Latent heat describes the amount of heat that is needed to evaporate the liquid; this heat comes from the liquid itself and the surrounding gas and surfaces. The greater the difference between the two temperatures, the greater the evaporative cooling effect. When the temperatures are the same, no net evaporation of water in air occurs; thus, there is no cooling effect. A simple example of natural evaporative cooling is perspiration, or sweat, which the body secretes in order to cool itself. An evaporative cooler is a device that cools air through the simple evaporation of water.[citation needed]
Radiative cooling
Radiative cooling is the process by which a body loses heat by radiation. It is an important effect in the Earth's atmosphere. In the case of the Earth-atmosphere system, it refers to the process by which long-wave (infrared) radiation is emitted to balance the absorption of short-wave (visible) energy from the Sun. Convective transport of heat and evaporative transport of latent heat both remove heat from the surface and redistribute it in the atmosphere, making it available for radiative transport at higher altitudes.[citation needed]
Laser cooling
Laser cooling refers to techniques in which atomic and molecular samples are cooled through the interaction with one or more laser light fields. The most common method of laser cooling is Doppler cooling. In Doppler cooling, the frequency of the laser light is tuned slightly below an electronic transition in the atom. Thus, the atoms would absorb more photons if they moved towards the light source, due to the Doppler effect. If an excited atom then emits a photon spontaneously, it will be accelerated. The result of the absorption and emission process is to reduce the speed of the atom. Eventually the mean velocity, and therefore the kinetic energy of the atoms, will be reduced. Since the temperature of an ensemble of atoms is a measure of the random internal kinetic energy, this is equivalent to cooling the atoms.
Sympathetic cooling is a process in which particles of one type cool particles of another type. Typically, atomic ions that can be directly laser-cooled are used to cool nearby ions or atoms. This technique allows cooling of ions and atoms that cannot be laser cooled directly.[citation needed]
Magnetic cooling
Magnetic evaporative cooling is a technique for lowering the temperature of a group of atoms. The process confines atoms using a magnetic field. Over time, individual atoms will become much more energetic than the others due to random collisions, and will escape—removing energy from the system and reducing the temperature of the remaining group. This process is similar to the familiar process by which standing water becomes water vapor.[citation needed]
Heat transfer in the human body
The principles of heat transfer in engineering systems can be applied to the human body in order to determine how the body transfers heat. Heat is produced in the body by the continuous metabolism of nutrients which provides energy for the systems of the body.[22] The human body must maintain a consistent internal temperature in order to maintain healthy bodily functions. Therefore, excess heat must be dissipated from the body to keep it from overheating. When a person engages in elevated levels of physical activity, the body requires additional fuel which increases the metabolic rate and the rate of heat production. The body must then use additional methods to remove the additional heat produced in order to keep the internal temperature at a healthy level.
Heat transfer by convection is driven by the movement of fluids over the surface of the body. This convective fluid can be either a liquid or a gas. For heat transfer from the outer surface of the body, the convection mechanism is dependent on the surface area of the body, the velocity of the air, and the temperature gradient between the surface of the skin and the ambient air.[23] The normal temperature of the body is approximately 37°C. Heat transfer occurs more readily when the temperature of the surroundings is significantly less than the normal body temperature. This concept explains why a person feels “cold” when not enough covering is worn when exposed to a cold environment. Clothing can be considered an insulator which provides thermal resistance to heat flow over the covered portion of the body.[24] This thermal resistance causes the temperature on the surface of the clothing to be less than the temperature on the surface of the skin. This smaller temperature gradient between the surface temperature and the ambient temperature will cause a lower rate of heat transfer than if the skin were not covered.
In order to ensure that one portion of the body is not significantly hotter than another portion, heat must be distributed evenly through the bodily tissues. Blood flowing through blood vessels acts as a convective fluid and helps to prevent any buildup of excess heat inside the tissues of the body. This flow of blood through the vessels can be modeled as pipe flow in an engineering system. The heat carried by the blood is determined by the temperature of the surrounding tissue, the diameter of the blood vessel, the thickness of the fluid, velocity of the flow, and the heat transfer coefficient of the blood. The velocity, blood vessel diameter, and the fluid thickness can all be related with the Reynolds Number, a dimensionless number used in fluid mechanics to characterize the flow of fluids.
Latent heat loss, also known as evaporative heat loss, accounts for a large fraction of heat loss from the body. When the core temperature of the body increases, the body triggers sweat glands in the skin to bring additional moisture to the surface of the skin. The liquid is then transformed into vapor which removes heat from the surface of the body.[25] The rate of evaporation heat loss is directly related to the vapor pressure at the skin surface and the amount of moisture present on the skin.[23] Therefore, the maximum of heat transfer will occur when the skin is completely wet. The body continuously loses water by evaporation but the most significant amount of heat loss occurs during periods of increased physical activity.
Other
A heat pipe is a passive device constructed in such a way that it acts as though it has extremely high thermal conductivity. Heat pipes use latent heat and capillary action to move heat, and can carry many times as much heat as a similar-sized copper rod. Originally invented for use in satellites, they have applications in personal computers. Another major use is for solar hot water panels.
A thermocouple is a junction between two different metals that produces a voltage related to a temperature difference. Thermocouples are a widely used type of temperature sensor for measurement and control, and can also be used to convert heat into electric power.
A thermopile is an electronic device that converts thermal energy into electrical energy. It is composed of thermocouples. Thermopiles do not measure the absolute temperature, but generate an output voltage proportional to a temperature difference. Thermopiles are widely used, e.g., they are the key component of infrared thermometers, such as those used to measure body temperature via the ear.[citation needed]
A thermoelectric cooler is a solid state electronic device that pumps (transfers) heat from one side of the device to the other when electrical current is passed through it. It is based on the Peltier effect.
A thermal diode or thermal rectifier is a device that preferentially passes heat in one direction: a "one-way valve" for heat.[citation needed]
See also
- Boiling
- Combined forced and natural convection
- Heat transfer physics
- Stefan–Boltzmann law
- Thermal contact conductance
- Thermal physics
- Thermal resistance in electronics
- Thermal science
References
- ^ Taylor, R.A., Socioeconomic impacts of heat transfer research, International Communications in Heat and Mass Transfer Volume 39, Issue 10, December 2012, Pages 1467–1473, http://www.sciencedirect.com/science/article/pii/S0735193312002199
- ^ Geankoplis, Christie John (2003). Transport processes and separation process principles : (includes unit operations) (4th ed. ed.). Upper Saddle River, NJ: Prentice Hall Professional Technical Reference. ISBN 0-13-101367-X.
{{cite book}}:|edition=has extra text (help) - ^ Lienhard, John H.,V; Lienhard, John H., V (2008). A Heat Transfer Textbook (3rd ed.). Cambridge, Massachusetts: Phlogiston Press. ISBN 978-0-9713835-3-1. OCLC 230956959.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b New Jersey Institute of Technology, Chemical Engineering Dept. "B.S. Chemical Engineering". NJIT. Retrieved 9 April 2011.
- ^ Welty, James R.; Wicks, Charles E.; Wilson, Robert Elliott (1976). Fundamentals of momentum, heat, and mass transfer (2 ed.). New York: Wiley. ISBN 978-0-471-93354-0. OCLC 2213384.
- ^ a b Faghri, Amir; Zhang, Yuwen; Howell, John (2010). Advanced Heat and Mass Transfer. Columbia, MO: Global Digital Press. ISBN 978-0-9842760-0-4.
- ^ a b c Abbott, J.M. Smith, H.C. Van Ness, M.M. (2005). Introduction to chemical engineering thermodynamics (7th ed. ed.). Boston ; Montreal: McGraw-Hill. ISBN 0-07-310445-0.
{{cite book}}:|edition=has extra text (help)CS1 maint: multiple names: authors list (link) - ^ "Thermal-FluidsPedia | Heat conduction".
- ^ Çengel, Yunus (2003). Heat Transfer: a practical approach. McGraw-Hill series in mechanical engineering. (2nd ed.). Boston: McGraw-Hill. ISBN 978-0-07-245893-0. OCLC 300472921. Retrieved 20 April 2009.
- ^ "Thermal-FluidsPedia | Convective heat transfer"
- ^ "Convection — Heat Transfer". Engineers Edge. Engineers Edge. Retrieved 20 April 2009.
- ^ "Thermal-FluidsPedia | Radiation"
- ^ Mojiri, A., Spectral beam splitting for efficient conversion of solar energy—A review, Renewable and Sustainable Energy Reviews Volume 28, December 2013, Pages 654–663
- ^ Taylor, R.A., Applicability of Nanofluids in High Flux Solar Collectors JOURNAL OF RENEWABLE AND SUSTAINABLE ENERGY 3, 023104, 2011
- ^ "Thermal-FluidsPedia | Mass transfer"
- ^ "Thermal-FluidsPedia | Multiphase systems"
- ^ Martin, Randy L. (29 July 2008). "R-Value Table". ColoradoENERGY.org. Windsor, Colorado: R. L. Martin & Associates, Inc. Retrieved 8 November 2010.
- ^ Bergman, Lavine, Incropera and DeWitt, Introduction to Heat Transfer (sixth edition), Wiley, 2011.
- ^ "EnergySavers: Tips on Saving Money & Energy at Home" (PDF). U.S. Department of Energy. Retrieved 2 March 2012.
- ^ Kuznetsov, A. V. (1996). "A Perturbation Solution for a Nonthermal Equilibrium Fluid Flow Through a Three-Dimensional Sensible Heat Storage Packed Bed". Journal of Heat Transfer. 118 (2): 508. doi:10.1115/1.2825881.
- ^ "Underground Thermal Energy Storage". Icax.co.uk. Retrieved 30 June 2012.
- ^ Hartman,Carl and Bibb, Lewis. "The Human body and its enemies: a textbook of physiology hygiene and sanitation", World Book Co., 1913, p.232.
- ^ a b Cengel, Yunus A. and Ghajar, Afshin J. "Heat and Mass Transfer: Fundamentals and Applications." , McGraw-Hill, 4th Edition, 2010.
- ^ Tao, Xiaoming. "Smart fibres, fabrics, and clothing" , Woodhead Publishing, 2001
- ^ Wilmore, Jack H., Costill, David L., Kenney, Larry, "Physiology of sport and exercise", Human Kinetics, 2008, p.256.
Further reading
- Frontiers in Heat and Mass Transfer
- Heat Transfer Engineering
- Experimental Heat Transfer
- International Journal of Heat and Mass Transfer
- ASME Journal of Heat Transfer
- Numerical Heat Transfer Part A
- Numerical Heat Transfer Part B
- Nanoscale and Microscale Thermophysical Engineering
- Journal of Enhanced Heat Transfer
External links
- Thermal-FluidsPedia - An online thermal fluids encyclopedia.
- Hyperphysics Article on Heat Transfer - Overview
- Interseasonal Heat Transfer - a practical example of how heat transfer is used to heat buildings without burning fossil fuels.
- Aspects of Heat Transfer, Cambridge University
- Thermal-Fluids Central
- Energy2D: Interactive Heat Transfer Simulations for Everyone
- Heat and Mass Transport Equation Sheet













