Niobium(V) ethoxide: Difference between revisions
No edit summary |
−Category:Organoniobium compounds; +Category:Niobium(V) compounds using HotCat: Organic, but not organometallic |
||
| (49 intermediate revisions by 17 users not shown) | |||
| Line 1: | Line 1: | ||
<!-- EDIT BELOW THIS LINE --> |
|||
Niobium (V) ethoxide, also known as Columbium Pentaethoxide or Niobium(V) Pentaethoxide, is an [[organometallic compound]] with a condensed formula of Nb(OC<sub>2</sub>H<sub>5</sub>)<sub>5</sub>. Niobium (V) Ethoxide is an organometallic compound containing mutiple [[bond]]s between ethyl and [[niobium]]. A molecule with bonds between a transition metal and a non-metal are classified within [[organometallic chemistry]], which have a variety of applications. Niobium (V) Ethoxide is commonly used in [[Chemical vapor deposition]] (CVD) <ref>Toshiro Maruyama1 and Tetsuya Kanagawa. Electrochromic Properties of Niobium Oxide Thin Films Prepared by Chemical Vapor Deposition. © 1994 ECS - The Electrochemical Society. Retreived November 15, 2012.</ref>. Niobium (V) Ethoxide's structure is closely related to that of [[Tantalum (V) Ethoxide]]. |
|||
{{Chembox |
{{Chembox |
||
| |
| Name = Niobium(V) Ethoxide |
||
| |
| ImageFile = Nb2(OEt)10.svg |
||
| |
| ImageSize = |
||
| |
| ImageAlt = |
||
| |
| ImageCaption = Skeletal structure of niobium(V) ethoxide |
||
| |
| SystematicName = |
||
| OtherNames = |
|||
| ImageSize1 = |
|||
|Section1={{Chembox Identifiers |
|||
| ImageAlt1 = |
|||
| |
| Abbreviations = |
||
| CASNo = 3236-82-6 |
|||
| ImageFile2 = |
|||
| PubChem = 520571 |
|||
| ImageSize2 = |
|||
| ChemSpiderID = 21241198 |
|||
| ImageAlt2 = |
|||
| EINECS = 221-795-2 |
|||
| ImageCaption2 = |
|||
| SMILES = CCO[Nb](OCC)(OCC)(OCC)OCC |
|||
| ImageFile3 = |
|||
| InChI = 1S/5C2H5O.Nb/c5*1-2-3;/h5*2H2,1H3;/q5*-1;+5 |
|||
| ImageSize3 = |
|||
| InChI_Ref=<ref>{{Cite web | url = http://www.chemspider.com/Chemical-Structure.21241198.html | publisher = ChemSpider | title = ChemSpider CSID:13600 | accessdate = 17 November 2012}}</ref> |
|||
| ImageAlt3 = |
|||
| StdInChIKey = PNCDAXYMWHXGON-UHFFFAOYSA-N |
|||
| ImageCaption3 = |
|||
| |
| Gmelin = |
||
}} |
|||
| ImageSize4 = |
|||
|Section2={{Chembox Properties |
|||
| ImageAlt4 = |
|||
| Formula = C<sub>10</sub>H<sub>25</sub>NbO<sub>5</sub> |
|||
| ImageCaption4 = |
|||
| MolarMass = 318.209 g mol<sup>−1</sup> |
|||
| ImageFileL1 = |
|||
| Appearance = Colourless liquid |
|||
| ImageSizeL1 = |
|||
| Density = 1.258 g cm<sup>−3</sup> |
|||
| ImageAltL1 = |
|||
| MeltingPtC = 5 |
|||
| ImageCaptionL1 = |
|||
| |
| MeltingPt_notes = |
||
| BoilingPtC = 203 |
|||
| ImageSizeR1 = |
|||
| |
| BoilingPt_notes = |
||
| LogP = |
|||
| ImageCaptionR1 = |
|||
| |
| VaporPressure = |
||
| |
| HenryConstant = |
||
| AtmosphericOHRateConstant = |
|||
| ImageAltL2 = |
|||
| pKa = |
|||
| ImageCaptionL2 = |
|||
| |
| pKb = |
||
| Solubility = N/A; reacts with water<ref name="water" /> }} |
|||
| ImageSizeR2 = |
|||
|Section3={{Chembox Structure |
|||
| ImageAltR2 = |
|||
| CrystalStruct = |
|||
| ImageCaptionR2 = |
|||
| Coordination = |
|||
| IUPACName = Niobium (V) Ethoxide |
|||
| MolShape = |
|||
| IUPACName_hidden = no |
|||
}} |
|||
| SystematicName = Monobutyltin Chloride |
|||
|Section4={{Chembox Thermochemistry |
|||
| OtherNames = {{Plainlist| |
|||
| DeltaHf = −1583.9 ± 2.7 kJ mol<sup>−1</sup><ref name="NIST">{{cite web |url=http://webbook.nist.gov/cgi/cbook.cgi?ID=C3236826&Units=SI&Mask=7 |title=Niobium(5+) ethanolate |website=webbook.nist.gov |access-date=November 15, 2012}}</ref> |
|||
*Niobium Ethylate |
|||
| DeltaHc = −6872.6 ± 1.7 kJ mol<sup>−1</sup><ref name="NIST" /> |
|||
*Niobium Ethoxide |
|||
| Entropy = |
|||
*Niobium(V) ethylate |
|||
| HeatCapacity = |
|||
*Niobum (V) ethoxide |
|||
}} |
|||
*Niobium Pentaethoxide |
|||
|Section7={{Chembox Hazards |
|||
*Pentaethoxyniobium(V) |
|||
| ExternalSDS = |
|||
*niobium(5+) ethanolate |
|||
| MainHazards = |
|||
*Columbium Pentaethoxide |
|||
| NFPA-H = 1 |
|||
*Niobium(V) Pentaethoxide <ref>http://www.chemicalbook.com/ChemicalProductProperty_EN_CB3759592.htm. Retrieved October 18,2012.</ref> }} |
|||
| NFPA-F = 1 |
|||
| Section1 = {{Chembox Identifiers |
|||
| NFPA-R = 1 |
|||
| Abbreviations = |
|||
| |
| NFPA-S = COR |
||
| GHSPictograms = {{GHS02}}{{GHS05}} |
|||
| CASNo_Comment = |
|||
| |
| GHSSignalWord = Danger |
||
| HPhrases = {{H-phrases|226|314}} |
|||
| CASNos = |
|||
| PPhrases = {{P-phrases|210|233|240|241|242|243|260|264|280|301+330+331|303+361+353|304+340|305+351+338|310|321|363|370+378|403+235|405|501}} |
|||
| CASOther = |
|||
| |
| FlashPtF = 97 |
||
| AutoignitionPtC = |
|||
| PubChem_Comment = |
|||
| |
| ExploLimits = |
||
| LD50 = |
|||
| PubChem5_Comment = |
|||
| |
| PEL = |
||
}} |
|||
| ChemSpiderID = 21241198 |
|||
|Section8={{Chembox Related |
|||
| ChemSpiderID_Comment = |
|||
| |
| OtherAnions = |
||
| OtherCations = |
|||
| ChemSpiderIDOther = |
|||
| OtherFunction = |
|||
| EINECS = 221-795-2 |
|||
| OtherFunction_label = |
|||
| EC-number = |
|||
| |
| OtherCompounds = |
||
}} |
|||
| UNNumber = |
|||
}} |
|||
| DrugBank = |
|||
'''Niobium(V) ethoxide''' is an metalorganic compound with formula Nb<sub>2</sub>(OC<sub>2</sub>H<sub>5</sub>)<sub>10</sub>. It is a colorless liquid that dissolves in some organic solvents but hydrolyzes readily.<ref name="water">W. M. Haynes. CRC Handbook of Chemistry and Physics, 93rd Edition. Physical Constants of Inorganic Compounds.</ref> It is mainly used for the [[sol-gel process]]ing of materials containing niobium oxides.<ref name=Ulrich/> |
|||
| KEGG = |
|||
| MeSHName = |
|||
| ChEBI = |
|||
| RTECS = |
|||
| ATCvet = |
|||
| ATCCode_prefix = |
|||
| ATCCode_suffix = |
|||
| ATC_Supplemental = |
|||
| SMILES = CCO[Nb](OCC)(OCC)(OCC)OCC |
|||
| InChI = 1S/5C2H5O.Nb/c5*1-2-3;/h5*2H2,1H3;/q5*-1;+5 <ref>{{Cite web |url=http://www.chemspider.com/Chemical-Structure.21241198.html|publisher=ChemSpider |title=ChemSpider CSID:13600 |accessdate=17 November 2012}}</ref> |
|||
| Beilstein = |
|||
| Gmelin = |
|||
| 3DMet =}} |
|||
| Section2 = {{Chembox Properties |
|||
| Formula = C<sub>10</sub>H<sub>25</sub>NbO<sub>5</sub> |
|||
| MolarMass = 318.209 g mol<sup>−1</sup> |
|||
| Appearance = colorless to yellow liquid <ref>http://www.sigmaaldrich.com/catalog/product/aldrich/760412?lang=en®ion=US. Retrieved October 18,2012.</ref> |
|||
| Density = 1.258 g/cm<sup>3</sup> |
|||
| MeltingPtC = 5 |
|||
| Melting_notes = |
|||
| BoilingPtC = 203 |
|||
| Boiling_notes = |
|||
| LogP = |
|||
| VaporPressure = |
|||
| HenryConstant = |
|||
| AtmosphericOHRateConstant = |
|||
| pKa = |
|||
| pKb = |
|||
| Sheet Resistance = |
|||
| Methacrylate Equiv Wt = |
|||
| Bulk Conductivity = }} |
|||
| Section3 = {{Chembox Structure |
|||
| CrystalStruct = |
|||
| Coordination = |
|||
| MolShape = }} |
|||
| Section4 = {{Chembox Thermochemistry |
|||
| Solubility = N/A; reacts with water <ref>W. M. Haynes. CRC Handbook of Chemistry and Physics, 93rd Edition. Physical Constants of Inorganic Compounds. Created 5/29/2012; Retrieved, November 15, 2012.</ref> |
|||
| SolubleOther = |
|||
| Solvent = |
|||
| DeltaHc = -6872.6 ± 1.7 kJ/mol |
|||
| DeltaHf = -1583.9 ± 2.7 kJ/mol <ref>http://webbook.nist.gov/cgi/cbook.cgi?ID=C3236826&Units=SI&Mask=7. Retrieved November 15, 2012.</ref> |
|||
| Entropy = |
|||
| HeatCapacity = }} |
|||
| Section5 = {{Chembox Pharmacology |
|||
| AdminRoutes = |
|||
| Bioavail = |
|||
| Metabolism = |
|||
| HalfLife = |
|||
| ProteinBound = |
|||
| Excretion = |
|||
| Legal_status = |
|||
| Legal_US = |
|||
| Legal_UK = |
|||
| Legal_AU = |
|||
| Legal_CA = |
|||
| PregCat = |
|||
| PregCat_AU = |
|||
| PregCat_US = }} |
|||
| Section6 = {{Chembox Explosive |
|||
| ShockSens = |
|||
| FrictionSens = |
|||
| ExplosiveV = |
|||
| REFactor = }} |
|||
| Section7 = {{Chembox Hazards |
|||
| ExternalMSDS = |
|||
| EUClass = |
|||
| EUIndex = |
|||
| MainHazards = |
|||
| NFPA-H = 1 |
|||
| NFPA-F = 1 |
|||
| NFPA-R = 1 |
|||
| NFPA-O = COR |
|||
| RPhrases = {{R10}},{{R34}} |
|||
| SPhrases = {{S26}},{{S36}},{{S37}},{{S39}},{{S45}} |
|||
| RSPhrases = |
|||
| FlashPt = 97<sup>o</sup>F |
|||
| Autoignition = |
|||
| ExploLimits = |
|||
| LD50 = |
|||
| PEL = }} |
|||
| Section8 = {{Chembox Related |
|||
| OtherAnions = |
|||
| OtherCations = |
|||
| OtherFunctn = |
|||
| Function = |
|||
| OtherCpds = }} |
|||
}} |
|||
==Structure== |
|||
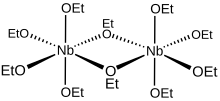
Metal alkoxides rarely adopt monomeric structures, and niobium(V) ethoxide is no exception. Early studies established that niobium alkoxides aggregate in solution as [[dimer (chemistry)|dimers]].<ref>{{cite journal|first1 = D. C.|last1 = Bradley|authorlink1 = Donald Charlton Bradley|first2 = C. E.|last2 = Holloway|title = Nuclear Magnetic Resonance Studies on Niobium and Tantalum Penta-alkoxides|journal = [[J. Chem. Soc. A]]|year = 1968|pages = 219–223|doi = 10.1039/J19680000219| s2cid=98638647 }}</ref> Subsequent [[crystallography|crystallographic analysis]] established that the methoxide and isopropoxides of niobium adopt bioctahedral structures.<ref name=Mehrota>{{cite book|first1 = Ram C.|last1 = Mehrotra|authorlink1 = Ram Charan Mehrotra|first2 = Anirudh|last2 = Singh|editor1-first = Kenneth D.|editor1-last = Karlin|chapter = Recent Trends in Metal Alkoxide Chemistry|title = Progress in Inorganic Chemistry|year = 1997|volume = 46|pages = 239–454|publisher = [[John Wiley & Sons]]|isbn = 9780470167045|doi = 10.1002/9780470166475.ch4|chapter-url = https://books.google.com/books?id=Pui4TU1yzYkC&pg=PA239}}</ref> From a geometric perspective, the ten ethoxide [[ligand]] oxygen atoms of the Nb<sub>2</sub>(OEt)<sub>10</sub> molecule in solution define a pair of octahedra sharing a common edge with the two niobium atoms located at their centres. From a bonding perspective, each niobium centre is surrounded octahedrally by four [[monodentate]] and two [[bridging ligand|bridging ethoxide ligands]]. The oxygen atoms of the bridging ethoxides are each bonded to both niobium centres, and these two ligands are [[Cis-trans isomerism#Coordination complexes|''cis'']] to one another within the [[coordination sphere]]. The formula [(EtO)<sub>4</sub>Nb(μ-OEt)]<sub>2</sub> more comprehensively represents this dimeric structure, though the simplified formula is commonly used for most purposes. |
|||
==Preparation and reactions== |
|||
Basic Summary |
|||
This compound is prepared by [[salt metathesis]] from [[niobium pentachloride]] (Et = C<sub>2</sub>H<sub>5</sub>): |
|||
:10 NaOEt + Nb<sub>2</sub>Cl<sub>10</sub> → Nb<sub>2</sub>(OC<sub>2</sub>H<sub>5</sub>)<sub>10</sub> + 10 NaCl |
|||
The most important reaction of niobium alkoxides is their hydrolysis to produce films and gels of niobium oxides.<ref name=Ulrich>U. Schubert "Sol–Gel Processing of Metal Compounds" Comprehensive Coordination Chemistry II 2003, Pages 629–656 Volume 7. {{doi|10.1016/B0-08-043748-6/06213-7}}</ref> Although these reactions are complex, they can be described by this simplified equation: |
|||
==Production== |
|||
:Nb<sub>2</sub>(OC<sub>2</sub>H<sub>5</sub>)<sub>10</sub> + 5 H<sub>2</sub>O → Nb<sub>2</sub>O<sub>5</sub> + 10 HOEt |
|||
The thermal decomposition of Nb(OC<sub>2</sub>H<sub>5</sub>)<sub>5</sub> begins above 325 – 350 °C. This can be observed with QMS as an increasing amount of ethanol and [[ethane]] released. [[Diethyl ether]], C<sub>2</sub>H<sub>5</sub>OC<sub>2</sub>H<sub>5</sub>, and [[niobium(V) oxide]] are the decomposition products released following an [[atomic layer deposition]] or [[chemical vapor deposition]] process. The decomposition reaction can be summarised as:<ref>{{cite thesis | title = Atomic Layer Deposition of High Permittivity Oxides: Film Growth and In Situ Studies | author = Rahtu, Antti | publisher = University of Helsinki | year = 2002 | hdl = 10138/21065 | isbn = 952-10-0646-3}}</ref> |
|||
One possible way to synthesize niobium ethoxide is by an electrochemical reaction. The reaction of ethanol with niobium plate as the sacrificial anode, stainless steel as the cathode, and tetraethylammonium chloride (TEAC) as the conductive additive yields niobium ethoxide.<ref>Ya-nan Cai. Electrochemical synthesis, characterization, and thermal properties of niobium ethoxide. Journal of Central South University of Technology. Volume 18, Number 1 (2011), 73-77, DOI: 10.1007/s11771-011-0661-2. |
|||
</ref> |
|||
:Nb<sub>2</sub>(OC<sub>2</sub>H<sub>5</sub>)<sub>10</sub> → Nb<sub>2</sub>O<sub>5</sub> + 5 O(C<sub>2</sub>H<sub>5</sub>)<sub>2</sub> |
|||
==Applications== |
|||
==References== |
|||
Group 5 (V, Nb and Ta) element-alkoxides can act as catalysts in the trans-esterification of ethylene-carbonate with methanol, ethanol and allyl alcohol. <ref>http://www.multivalent.co.uk/chemicals/applications.php?app_sector_id=007&chemical_id=34. Retrieved October 18, 2012.</ref> |
|||
{{Reflist}} |
|||
{{Niobium compounds}} |
|||
==Reactions== |
|||
The stoichiometric reaction between salicylaldoxime and niobium ethoxide in toluene at room temperature results in the formation of [Nb<sub>2</sub>O(C<sub>7</sub>H<sub>5</sub>NO<sub>2</sub>)<sub>2</sub>(C<sub>2</sub>H<sub>5</sub>O)<sub>4</sub>]: <ref>Mahdi Mirzaee (2011). The crystal structure of μ-Oxo-bis{diethoxy[salicylaldoximato(2-)]-tantalum(V)} Comptes Rendus Chimie. Volume 14, Issue 10.</ref> |
|||
C<sub>6</sub>H<sub>4</sub>4CH=NOH-2-OH + C<sub>10</sub>H<sub>25</sub>NbO<sub>5</sub> → [Nb<sub>2</sub>O(C<sub>7</sub>H<sub>5</sub>NO<sub>2</sub>)<sub>2</sub>(C<sub>2</sub>H<sub>5</sub>O)<sub>4</sub>] |
|||
The thermal decomposition of Nb (OC<sub>2</sub>H<sub>5</sub>)<sub>5</sub> begins above 325 - 350°C. This can be observed with QMS as an increasing amount of ethanol and ethane released. Diethyl ether (O(C<sub>2</sub>H<sub>5</sub>)<sub>2</sub>) is one of the decomposition byproducts, though it can also be released in some extent from a [[Atomic Layer Deposition]] (ALD) reaction. The decomposition reaction can be proposed as:<ref>Antti Rahtu. Atomic Layer Deposition of High Permittivity Oxides: |
|||
Film Growth and In Situ Studies. HELSINKI 2002. Retrieved November 15, 2012</ref>: |
|||
2 Nb(OC<sub>2</sub>H<sub>5</sub>)<sub>5</sub>(g) → Nb<sub>2</sub>O<sub>5</sub>(s) + O(C<sub>2</sub>H<sub>5</sub>)<sub>2</sub> (g). |
|||
==Physical properties== |
|||
Niobium ethoxide is an organometallic compound that is a clear to yellow liquid. It has a density of 1.268 g cm<sup>-1</sup> <ref>http://mastersearch.chemexper.com/cheminfo/servlet/org.dbcreator.MainServlet?query=entry._entryID%3D1766126&target=entry&action=PowerSearch&searchInfo=quicksearch&format=ccd&searchValue=3236826&options=brandqtyoffer. Retrieved October 18, 2012.</ref>. It also has a melting point of 5-6<sup>o</sup>C, a boiling point of 140-142<sup>o</sup>C, and a refraction index of 1.516. Niobium Ethoxide is also very flammable. Being a group 5 element-alkoxide, its properties, reactivity, and applications can be compared to that of Tantalum (V) Ethoxide. |
|||
==Safety== |
|||
:P301 + P330 + P331: IF SWALLOWED: rinse mouth. Do NOT induce vomiting |
|||
:P280: Wear eye protection/face protection |
|||
:P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing |
|||
:P310: Immediately call a POISON CENTER or doctor/physician |
|||
:P210: Keep away from heat/sparks/open flames/hot surfaces. - No smoking <ref>http://www.chemexper.com/cheminfo/servlet/org.dbcreator.MainServlet?query=entry._entryID%3D20394&target=entry&action=PowerSearch&format=google2008. Retrieved November 15, 2012.</ref> |
|||
==References== |
|||
[[Category:Ethoxides]] |
|||
{{Reflist|2}} |
|||
[[Category:Niobium(V) compounds]] |
|||
Latest revision as of 10:06, 20 June 2022
 Skeletal structure of niobium(V) ethoxide
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.019.814 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C10H25NbO5 | |
| Molar mass | 318.209 g mol−1 |
| Appearance | Colourless liquid |
| Density | 1.258 g cm−3 |
| Melting point | 5 °C (41 °F; 278 K) |
| Boiling point | 203 °C (397 °F; 476 K) |
| N/A; reacts with water[2] | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−1583.9 ± 2.7 kJ mol−1[3] |
Std enthalpy of
combustion (ΔcH⦵298) |
−6872.6 ± 1.7 kJ mol−1[3] |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H226, H314 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P370+P378, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 36 °C; 97 °F; 309 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Niobium(V) ethoxide is an metalorganic compound with formula Nb2(OC2H5)10. It is a colorless liquid that dissolves in some organic solvents but hydrolyzes readily.[2] It is mainly used for the sol-gel processing of materials containing niobium oxides.[4]
Structure[edit]
Metal alkoxides rarely adopt monomeric structures, and niobium(V) ethoxide is no exception. Early studies established that niobium alkoxides aggregate in solution as dimers.[5] Subsequent crystallographic analysis established that the methoxide and isopropoxides of niobium adopt bioctahedral structures.[6] From a geometric perspective, the ten ethoxide ligand oxygen atoms of the Nb2(OEt)10 molecule in solution define a pair of octahedra sharing a common edge with the two niobium atoms located at their centres. From a bonding perspective, each niobium centre is surrounded octahedrally by four monodentate and two bridging ethoxide ligands. The oxygen atoms of the bridging ethoxides are each bonded to both niobium centres, and these two ligands are cis to one another within the coordination sphere. The formula [(EtO)4Nb(μ-OEt)]2 more comprehensively represents this dimeric structure, though the simplified formula is commonly used for most purposes.
Preparation and reactions[edit]
This compound is prepared by salt metathesis from niobium pentachloride (Et = C2H5):
- 10 NaOEt + Nb2Cl10 → Nb2(OC2H5)10 + 10 NaCl
The most important reaction of niobium alkoxides is their hydrolysis to produce films and gels of niobium oxides.[4] Although these reactions are complex, they can be described by this simplified equation:
- Nb2(OC2H5)10 + 5 H2O → Nb2O5 + 10 HOEt
The thermal decomposition of Nb(OC2H5)5 begins above 325 – 350 °C. This can be observed with QMS as an increasing amount of ethanol and ethane released. Diethyl ether, C2H5OC2H5, and niobium(V) oxide are the decomposition products released following an atomic layer deposition or chemical vapor deposition process. The decomposition reaction can be summarised as:[7]
- Nb2(OC2H5)10 → Nb2O5 + 5 O(C2H5)2
References[edit]
- ^ "ChemSpider CSID:13600". ChemSpider. Retrieved 17 November 2012.
- ^ a b W. M. Haynes. CRC Handbook of Chemistry and Physics, 93rd Edition. Physical Constants of Inorganic Compounds.
- ^ a b "Niobium(5+) ethanolate". webbook.nist.gov. Retrieved November 15, 2012.
- ^ a b U. Schubert "Sol–Gel Processing of Metal Compounds" Comprehensive Coordination Chemistry II 2003, Pages 629–656 Volume 7. doi:10.1016/B0-08-043748-6/06213-7
- ^ Bradley, D. C.; Holloway, C. E. (1968). "Nuclear Magnetic Resonance Studies on Niobium and Tantalum Penta-alkoxides". J. Chem. Soc. A: 219–223. doi:10.1039/J19680000219. S2CID 98638647.
- ^ Mehrotra, Ram C.; Singh, Anirudh (1997). "Recent Trends in Metal Alkoxide Chemistry". In Karlin, Kenneth D. (ed.). Progress in Inorganic Chemistry. Vol. 46. John Wiley & Sons. pp. 239–454. doi:10.1002/9780470166475.ch4. ISBN 9780470167045.
- ^ Rahtu, Antti (2002). Atomic Layer Deposition of High Permittivity Oxides: Film Growth and In Situ Studies (Thesis). University of Helsinki. hdl:10138/21065. ISBN 952-10-0646-3.

