Ozone layer: Difference between revisions
rv |
|||
| Line 35: | Line 35: | ||
== Ozone depletion== |
== Ozone depletion== |
||
{{main|Ozone depletion}} |
{{main|Ozone depletion}} |
||
The ozone layer can be depleted by free radical catalysts, including nitric oxide (NO), |
The ozone layer can be depleted by free radical catalysts, including nitric oxide (NO), hydroxyl (OH), atomic [[chlorine]] (Cl), and atomic [[bromine]] (Br). While there are natural sources for all of these species, the concentrations of chlorine and bromine have increased markedly in recent years due to the release of large quantities of manmade organohalogen compounds, especially [[chlorofluorocarbon]]s (CFCs) and bromofluorocarbons. These highly stable compounds are capable of surviving the rise to the [[stratosphere]], where Cl and Br [[Radical (chemistry)|radicals]] are liberated by the action of ultraviolet light. Each radical is then free to initiate and catalyze a chain reaction capable of breaking down over 10,000 ozone molecules. Ozone levels, over the [[northern hemisphere]], have been dropping by 4% per decade. Over approximately 5% of the Earth's surface, around the north and south poles, much larger (but seasonal) declines have been seen; these are the [[ozone hole]]s. |
||
=== Regulation === |
=== Regulation === |
||
Revision as of 07:32, 13 April 2007
The ozone layer, or ozonosphere layer (very rarely used term), is the part of the Earth's atmosphere which contains relatively high concentrations of ozone (O3). "Relatively high" means a few parts per million—much higher than the concentrations in the lower atmosphere but still small compared to the main components of the atmosphere. It is mainly located in the lower portion of the stratosphere from approximately 15 km to 35 km above Earth's surface, though the thickness varies seasonally and geographically.[1] The ozone layer was discovered in 1913 by the French physicists Charles Fabry and Henri Buisson. Its properties were explored in detail by the British meteorologist G. M. B. Dobson, who devised a simple spectrophotometer that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958, Dobson established a worldwide network of ozone monitoring stations which continues to operate today. The "Dobson unit", a convenient measure of the total amount of ozone in a column overhead, is named in his honor. It is interesting to note that ozone gas is a pollutant at lower levels and cause severe problems like oedema, hemorrage etc.
Ultraviolet light and ozone

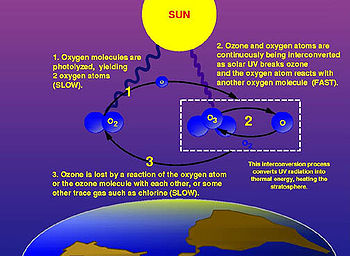
Although the concentration of ozone in the ozone layer is very small, it is vitally important to life because it absorbs biologically harmful ultraviolet (UV) radiation emitted from the Sun. UV radiation is divided into three categories, based on its wavelength; these are referred to as UV-A, UV-B, and UV-C. UV-C, which would be very harmful to humans, is entirely screened out by ozone at around 35 km altitude. UV-B radiation can be harmful to the skin and is the main cause of sunburn; excessive exposure can also cause genetic damage, resulting in problems such as skin cancer. The ozone layer is very effective at screening out UV-B; for radiation with a wavelength of 290 nm, the intensity at Earth's surface is 350 million times weaker than at the top of the atmosphere. Nevertheless, some UV-B reaches the surface. Most UV-A reaches the surface; this radiation is significantly less harmful, although it can potentially cause genetic damage.
Depletion of the ozone layer allows more of the UV radiation, and particularly the more harmful wavelengths, to reach the surface, causing increased genetic damage to living organisms.
DNA sensitivity to UV

To appreciate how important this ultraviolet radiation screening is, we can consider a characteristic of radiation damage called an action spectrum. An action spectrum gives us a measure of the relative effectiveness of radiation in generating a certain biological response over a range of wavelengths. This response might be erythema (sunburn), changes in plant growth, or changes in molecular DNA. There is much greater probability of DNA damage by UV radiation at various wavelengths. Fortunately, where DNA is easily damaged, such as by wavelengths shorter than 290 nm, ozone strongly absorbs UV. At the longer wavelengths where ozone absorbs weakly, DNA damage is less likely. If there was a 10% decrease in ozone, the amount of DNA damaging UV increases, in this case, by about 22%. Considering that DNA damage can lead to maladies like skin cancer, it is clear that this absorption of the sun's ultraviolet radiation by ozone is critical for our well being.
Distribution of ozone in the stratosphere

The "thickness" of the ozone layer—that is, the total amount of ozone in a column overhead—varies by a large factor worldwide, being in general smaller near the equator and larger as one moves towards the poles. It also varies with season, being in general thicker during the spring and thinner during the autumn. The reasons for this latitude and seasonal dependence are complicated, involving atmospheric circulation patterns as well as solar intensity.
Since stratospheric ozone is produced by solar UV radiation, one might expect to find the highest ozone levels over the tropics and the lowest over polar regions. The same argument would lead one to expect the highest ozone levels in the summer and the lowest in the winter. The observed behavior is very different: most of the ozone is found in the mid-to-high latitudes of the northern and southern hemispheres, and the highest levels are found in the spring, not summer, and the lowest in the autumn, not winter. During winter, the ozone layer actually increases in depth. This puzzle is explained by the prevailing stratospheric wind patterns, known as the Brewer-Dobson circulation. While most of the ozone is indeed created over the tropics, the stratospheric circulation then transports it poleward and downward to the lower stratosphere of the high latitudes.

The ozone layer is higher in altitude in the tropics, and lower in altitude in the extratropics, especially in the polar regions. This altitude variation of ozone results from the slow circulation that lifts the ozone-poor air out of the troposphere into the stratosphere. As this air slowly rises in the tropics, ozone is produced by the overhead sun which photolyzes oxygen molecules. As this slow circulation bends towards the mid-latitudes, it carries the ozone-rich air from the tropical middle stratosphere to the mid-and-high latitudes lower stratosphere. The high ozone concentrations at high latitudes are due to the accumulation of ozone at lower altitudes.
The Brewer-Dobson circulation moves very slowly. The time needed to lift an air parcel from the tropical tropopause near 16 km (50,000 feet) to 20 km is about 4-5 months (about 30 feet per day). Even though ozone in the lower tropical stratosphere is produced at a very slow rate, the lifting circulation is so slow that ozone can build up to relatively high levels by the time it reaches 26 km.
Ozone amounts over the continental United States (25°N to 49°N) are highest in the northern spring (April and May). These ozone amounts fall over the course of the summer to their lowest amounts in October, and then rise again over the course of the winter. Again, wind transport of ozone is principally responsible for the seasonal evolution of these higher latitude ozone patterns.
The total column amount of ozone generally increases as we move from the tropics to higher latitudes in both hemispheres. However, the overall column amounts are greater in the northern hemisphere high latitudes than in the southern hemisphere high latitudes. In addition, while the highest amounts of column ozone over the Arctic occur in the northern spring (March-April), the opposite is true over the Antarctic, where the lowest amounts of column ozone occur in the southern spring (September-October). Indeed, the highest amounts of column ozone anywhere in the world are found over the Arctic region during the northern spring period of March and April. The amounts then decrease over the course of the northern summer. Meanwhile, the lowest amounts of column ozone anywhere in the world are found over the Antarctic in the southern spring period of September and October, owing to the ozone hole phenomenon.
Ozone depletion
The ozone layer can be depleted by free radical catalysts, including nitric oxide (NO), hydroxyl (OH), atomic chlorine (Cl), and atomic bromine (Br). While there are natural sources for all of these species, the concentrations of chlorine and bromine have increased markedly in recent years due to the release of large quantities of manmade organohalogen compounds, especially chlorofluorocarbons (CFCs) and bromofluorocarbons. These highly stable compounds are capable of surviving the rise to the stratosphere, where Cl and Br radicals are liberated by the action of ultraviolet light. Each radical is then free to initiate and catalyze a chain reaction capable of breaking down over 10,000 ozone molecules. Ozone levels, over the northern hemisphere, have been dropping by 4% per decade. Over approximately 5% of the Earth's surface, around the north and south poles, much larger (but seasonal) declines have been seen; these are the ozone holes.
Regulation
On January 23, 1978, Sweden became the first nation to ban CFC-containing aerosol sprays that are thought to damage the ozone layer. A few other countries, including the United States, Canada, and Norway, followed suit later that year, but the European Community rejected an analogous proposal. Even in the U.S., chlorofluorocarbons continued to be used in other applications, such as refrigeration and industrial cleaning, until after the discovery of the Antarctic ozone hole in 1985. After negotiation of an international treaty (the Montreal Protocol), CFC production was sharply limited beginning in 1987 and phased out completely by 1996.
On August 2, 2003, scientists announced that the depletion of the ozone layer may be slowing down due to the international ban on CFCs. [2] Three satellites and three ground stations confirmed that the upper atmosphere ozone depletion rate has slowed down significantly during the past decade. The study was organized by the American Geophysical Union. Some breakdown can be expected to continue due to CFCs used by nations which have not banned them, and due to gases which are already in the stratosphere. CFCs have very long atmospheric lifetimes, ranging from 50 to over 100 years, so the final recovery of the ozone layer is expected to require several lifetimes.
Compounds containing C–H bonds are being designed to replace the function of CFC's (such as HFC), since these compounds are more reactive and less likely to survive long enough in the atmosphere to reach the stratosphere where they could affect the ozone layer.
- ^ "Science: Ozone Basics". Retrieved 2007-01-29.
- ^ Independent Online (2006-09-23). "Independent Online". Retrieved 2006-09-23.
{{cite web}}: Check date values in:|date=(help)
