Mitragynine (version 2): Difference between revisions
Iridescent (talk | contribs) m Cleanup & typo fixing using AWB |
replaced structural image with ACS compliant raster |
||
| Line 3: | Line 3: | ||
! {{chembox header}}| '''Mitragynine''' <!-- replace if not identical with the article name --> |
! {{chembox header}}| '''Mitragynine''' <!-- replace if not identical with the article name --> |
||
|- |
|- |
||
| align="center" colspan="2" bgcolor="#ffffff" | [[Image: |
| align="center" colspan="2" bgcolor="#ffffff" | [[Image:Mitragynine.png|200px|Mitragynine]] <!-- replace if not identical with the pagename --> |
||
|- |
|- |
||
| [[IUPAC nomenclature|Systematic name]] |
| [[IUPAC nomenclature|Systematic name]] |
||
Revision as of 04:03, 6 January 2008
| Mitragynine | |
|---|---|
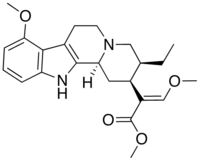
| |
| Systematic name | Mitragynine |
| Chemical formula | C23H30N2O4 |
| Molecular mass | 398.2206 g/mol |
| Density | x.xxx g/cm³ |
| Melting point | 39 - 41 °C |
| Melting point of HCl Salt | 117.22 °C |
| Boiling point | 110 - 115.6 °C |
| CAS number | [4098-40-2] |
| SMILES | xxxxx |
| Disclaimer and references | |
Mitragynine, an indole alkaloid, is the most abundant active alkaloid in the plant Mitragyna speciosa, commonly known as Kratom. In small doses its activity is reported to be stimulant like, while in higher doses more opiate like. These effects agree with the profile of mitragynine's actions developed by study of its action on the brain. It has been shown to be adrenergic (like some related alkaloids from yohimbe) at lower doses while at higher doses mitragynine acts on the mu and delta opiate receptors. The mu opiate receptors are responsible for the enjoyable effects of the opiates, analgesia, and physical dependence. Its potential for treating drug addiction, perhaps in combination with ibogaine, is being investigated. It is orally active.
Mitragynine was isolated in 1907 by D. Hooper, a process repeated in 1921 by E. Field who gave the alkaloid its name. Its structure was first fully determined in 1964 by D. Zacharias, R. Rosenstein and E. Jeffrey.
It is structurally related to both the yohimbe alkaloids and, more distantly, voacangine. It is even more distantly related to other tryptamine-based psychedelic drugs such as psilocybin or LSD. Chemically, mitragynine is 9-methoxy-corynantheidine. Mitragynine acts as a fairly selective δ-opioid agonist with little affinity for μ or κ receptors.
Chemical traits
Physically the freebase is a white, amorphous powder. It is soluble in alcohol, chloroform and acetic acid.
References
- Takayama H.; Maeda M.; Ohbayashi S.; Kitajima M.; Sakai S.-i.; Aimi N. (1995). "The First Total Synthesis of (-)-Mitragynine, An Analgesic Indole Alkaloid in Mitragyna speciosa". Tetrahedron Letters. 36 (51): 9337–9340. doi:10.1016/0040-4039(95)02022-H.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
