Fluorescence in situ hybridization: Difference between revisions
Aaronjhill (talk | contribs) No edit summary |
Image cleanup and formatting. Minor copyedits. |
||
| Line 1: | Line 1: | ||
{{Refimprove|date=January 2009}} |
{{Refimprove|date=January 2009}} |
||
[[Image:Bcrablmet.jpg|right|thumb|A metaphase cell positive for the ''bcr/abl'' rearrangement (associated with [[chronic myelogenous leukemia]]) using FISH. The chromosomes can be seen in blue. The chromosome that is labeled with green ''and'' red spots (up left) is the one where the wrong rearrangement is present.]] |
|||
'''FISH''' ([[fluorescence]] ''[[in situ]]'' [[hybridisation (molecular biology)|hybridization]]) is a [[cytogenetics|cytogenetic]] technique used to detect and localize the presence or absence of specific [[DNA]] [[DNA sequence|sequences]] on [[chromosome]]s. FISH uses [[hybridization probe|fluorescent probes]] that bind to only those parts of the chromosome with which they show a high degree of sequence similarity. [[Fluorescence microscopy]] can be used to find out where the fluorescent probe bound to the chromosomes. FISH is often used for finding specific features in DNA for use in genetic counseling, medicine, and species identification. FISH can also be used to detect and localize specific [[mRNA]]s within tissue samples. In this context, it can help define the spatial-temporal patterns of [[gene expression]] within cells and tissues. |
|||
== Probes == |
== Probes == |
||
[[Image:Urovysion on Duet.png|thumb| |
[[Image:Urovysion on Duet.png|thumb|right|Urothelial cells marked with four different probes.]] |
||
Probes are often derived from fragments of DNA that were isolated, purified, and amplified for use in the Human Genome Project. The size of the human genome is so large, compared to the length that could be sequenced directly, that it was necessary to divide the genome into fragments. The fragments were added into a framework that made it possible to use bacteria to replicate the fragments. The fragments were put into order by analyzing size-exclusion separation of enzymatically-digested fragments. Clonal populations of bacteria, each population maintaining a single artificial chromosome, are stored in various laboratories around the world. The artificial chromosomes ([[bacterial artificial chromosome|BAC]]) can be grown, extracted, and labeled, in any lab. These fragments are on the order of 100 thousand base-pairs, and are the basis for most FISH probes. |
Probes are often derived from fragments of DNA that were isolated, purified, and amplified for use in the Human Genome Project. The size of the human genome is so large, compared to the length that could be sequenced directly, that it was necessary to divide the genome into fragments. The fragments were added into a framework that made it possible to use bacteria to replicate the fragments. The fragments were put into order by analyzing size-exclusion separation of enzymatically-digested fragments. Clonal populations of bacteria, each population maintaining a single artificial chromosome, are stored in various laboratories around the world. The artificial chromosomes ([[bacterial artificial chromosome|BAC]]) can be grown, extracted, and labeled, in any lab. These fragments are on the order of 100 thousand base-pairs, and are the basis for most FISH probes. |
||
=== Preparation and Hybridization Process === |
=== Preparation and Hybridization Process === |
||
| ⚫ | |||
[[Image:FISH (Fluorescent In Situ Hybridization).jpg|thumb|right |
[[Image:FISH (Fluorescent In Situ Hybridization).jpg|thumb|right|Scheme of the principle of the FISH Experiment to localize a gene in the nucleus.]] |
||
First, a probe is constructed. The probe must be large enough to hybridize specifically with its target but not so large as to impede the hybridization process. The probe is [[Fluorescent tag|tagged]] directly with [[fluorophore]]s, with targets for [[antibodies]] or with [[biotin]]. Tagging can be done in various ways, such as [[nick translation]], or [[PCR]] using tagged [[nucleotide]]s. |
First, a probe is constructed. The probe must be large enough to hybridize specifically with its target but not so large as to impede the hybridization process. The probe is [[Fluorescent tag|tagged]] directly with [[fluorophore]]s, with targets for [[antibodies]] or with [[biotin]]. Tagging can be done in various ways, such as [[nick translation]], or [[PCR]] using tagged [[nucleotide]]s. |
||
| Line 20: | Line 21: | ||
=== Fiber FISH === |
=== Fiber FISH === |
||
In an alternative technique to interphase or metaphase preparations, fiber FISH, [[interphase]] chromosomes are attached to a slide in such a way that they are stretched out in a straight line, rather than being tightly coiled, as in conventional FISH, or adopting a random conformation, as in interphase FISH. This is accomplished by applying mechanical [[Shearing (physics)|shear]] along the length of the slide, either to cells that have been fixed to the slide and then [[Lysis|lyse]]d, or to a solution of purified DNA. A technique known as [[chromosome combing]] is increasingly used for this purpose. The extended conformation of the chromosomes allows dramatically higher resolution - even down to a few [[kilobase]]s. The preparation of fiber FISH samples, although conceptually simple, is a rather skilled art, and only specialized laboratories use the technique routinely. |
In an alternative technique to interphase or metaphase preparations, fiber FISH, [[interphase]] chromosomes are attached to a slide in such a way that they are stretched out in a straight line, rather than being tightly coiled, as in conventional FISH, or adopting a random conformation, as in interphase FISH. This is accomplished by applying mechanical [[Shearing (physics)|shear]] along the length of the slide, either to cells that have been fixed to the slide and then [[Lysis|lyse]]d, or to a solution of purified DNA. A technique known as [[chromosome combing]] is increasingly used for this purpose. The extended conformation of the chromosomes allows dramatically higher resolution - even down to a few [[kilobase]]s. The preparation of fiber FISH samples, although conceptually simple, is a rather skilled art, and only specialized laboratories use the technique routinely. |
||
=== Variations on |
=== Variations on probes and analysis=== |
||
| ⚫ | |||
FISH is a very general technique. It is often arbitrarily divided into more specific categories based on application, but each category is similar in that, in a chemical sense, the technique is the same -- hybridization is the common denominator. The differences between the various FISH techniques are usually due to the construction and content of the fluorescently-labeled DNA probe. The size, overlap, colour, and mixture of the probes make possible all FISH techniques. |
FISH is a very general technique. It is often arbitrarily divided into more specific categories based on application, but each category is similar in that, in a chemical sense, the technique is the same -- hybridization is the common denominator. The differences between the various FISH techniques are usually due to the construction and content of the fluorescently-labeled DNA probe. The size, overlap, colour, and mixture of the probes make possible all FISH techniques. |
||
| ⚫ | |||
Probe size is important because longer probes hybridize more specifically than shorter probes. The overlap defines the resolution of detectable features. If the goal of an experiment is to detect the breakpoint of a [[translocation]], then the overlap of the probes — the degree to which one DNA sequence is contained in the adjacent probes — defines the minimum window in which the breakpoint occurs. |
Probe size is important because longer probes hybridize more specifically than shorter probes. The overlap defines the resolution of detectable features. If the goal of an experiment is to detect the breakpoint of a [[translocation]], then the overlap of the probes — the degree to which one DNA sequence is contained in the adjacent probes — defines the minimum window in which the breakpoint occurs. |
||
The mixture of probes determines the type of feature the probe can detect. Probes that hybridize along an entire chromosome are used to count the number of a certain chromosome, show translocations, or identify extra-chromosomal fragments of [[chromatin]]. This is often called "whole-chromosome painting." |
The mixture of probes determines the type of feature the probe can detect. Probes that hybridize along an entire chromosome are used to count the number of a certain chromosome, show translocations, or identify extra-chromosomal fragments of [[chromatin]]. This is often called "whole-chromosome painting." If every possible probe is used, every chromosome, (in essence the whole genome) would be marked fluorescently, which would not be particularly useful for determining features of individual sequences. A mixture of smaller probes can be created that are specific to a particular region (locus) of DNA; these mixtures are used to detect [[Genetic deletion|deletion mutations]]. When combined with a specific colour, a locus-specific probe mixture is used to detect very specific translocations. Special locus-specific probe mixtures are often used to count chromosomes, by binding to the [[centromeric]] regions of chromosomes, which are unique enough to identify each chromosome (with the exception of [[Chromosome 13]], [[Chromosome 14|14]] [[Chromosome 21|21]], [[Chromosome 22|22]].) |
||
If every possible probe is used, every chromosome, (in essence the whole genome) would be marked fluorescently, which would not be particularly useful for determining features of individual sequences. A mixture of smaller probes can be created that are specific to a particular region (locus) of DNA; these mixtures are used to detect [[Genetic deletion|deletion mutations]]. When combined with a specific colour, a locus-specific probe mixture is used to detect very specific translocations. Special locus-specific probe mixtures are often used to count chromosomes, by binding to the [[centromeric]] regions of chromosomes, which are unique enough to identify each chromosome (with the exception of [[Chromosome 13]], [[Chromosome 14|14]] [[Chromosome 21|21]], [[Chromosome 22|22]].) |
|||
Because modern microscopes can detect a range of colours in fluorescent dyes, each human chromosome can be identified (M-FISH) using whole-chromosome probe mixtures and a variety of colours. There are currently twice as many chromosomes as fluorescent dye colours. However, ratios of probe mixtures can be used to create additional colours. As with [[comparative genomic hybridization]], the probe mixture for the secondary colours is created by mixing the correct ratio of two sets of differently-labeled probes for the same chromosome. Differently-coloured probes can be used for the detection of translocations. Several techniques exploit the resolution limitations of microscopes to resolve spatial distributions of dye below a few hundred [[nanometer]]s. Colours that are adjacent appear to overlap, and a secondary colour is observed. |
Because modern microscopes can detect a range of colours in fluorescent dyes, each human chromosome can be identified (M-FISH) using whole-chromosome probe mixtures and a variety of colours. There are currently twice as many chromosomes as fluorescent dye colours. However, ratios of probe mixtures can be used to create additional colours. As with [[comparative genomic hybridization]], the probe mixture for the secondary colours is created by mixing the correct ratio of two sets of differently-labeled probes for the same chromosome. Differently-coloured probes can be used for the detection of translocations. Several techniques exploit the resolution limitations of microscopes to resolve spatial distributions of dye below a few hundred [[nanometer]]s. Colours that are adjacent appear to overlap, and a secondary colour is observed. |
||
| Line 51: | Line 51: | ||
=== Lab-on-a-chip and FISH === |
=== Lab-on-a-chip and FISH === |
||
| ⚫ | |||
Although interphase fluorescence in situ hybridization (FISH) is a sensitive diagnostic tool used for the detection of chromosomal abnormalities on cell-by-cell basis, the cost-per-test and the technical complexity of current FISH protocols has inhibited its widespread utilization. Lab-on-a-chip or microfluidic devices, incorporate networks of microchannels that can miniaturize, integrate and automate conventional analytical techniques onto chip-style platforms. Since microchannels permit sophisticated levels of fluid control (down to picolitres), these devices can reduce analysis times, lower reagent consumption, and minimize human intervention. |
Although interphase fluorescence in situ hybridization (FISH) is a sensitive diagnostic tool used for the detection of chromosomal abnormalities on cell-by-cell basis, the cost-per-test and the technical complexity of current FISH protocols has inhibited its widespread utilization. Lab-on-a-chip or microfluidic devices, incorporate networks of microchannels that can miniaturize, integrate and automate conventional analytical techniques onto chip-style platforms. Since microchannels permit sophisticated levels of fluid control (down to picolitres), these devices can reduce analysis times, lower reagent consumption, and minimize human intervention. |
||
Currently, FISH has been performed on glass microfluidic platforms that standardize much of the protocol offering repeatable results that are accurate, cost-effective and easier to obtain in a clinical setting. |
Currently, FISH has been performed on glass microfluidic platforms that standardize much of the protocol offering repeatable results that are accurate, cost-effective and easier to obtain in a clinical setting. |
||
| ⚫ | |||
| ⚫ | |||
Compared to conventional FISH methods, these first implementations of on-chip FISH provide a 10-fold higher throughput and a 10-fold reduction in the cost of testing, enabling the simultaneous assessment of several chromosomal abnormalities or patients.<ref>{{Cite journal| doi = 10.1049/iet-nbt:20060021| volume = 1| issue = 3| pages = 27-35| last = Sieben| first = V.J.| coauthors = C.S. Debes Marun, P.M. Pilarski, G.V. Kaigala, L.M. Pilarski, C.J. Backhouse| title = FISH and chips: chromosomal analysis on microfluidic platforms| journal = IET Nanobiotechnology| accessdate = 2009-01-26| date = 2007-06| url = http://link.aip.org/link/?NBT/1/27/1}}</ref> It is increasingly essential that diagnostic tests determine the type and extent of chromosomal abnormalities for more informed diagnosis and for appropriate choice of treatment strategies. Since the on-chip FISH technique is 10-20 times more cost-effective than conventional methods, and can be fully integrated and automated<ref>{{Cite journal| doi = 10.1039/b812443d| volume = 8| issue = 12| pages = 2151-2156| last = Sieben| first = V.J.| coauthors = C.S. Debes-Marun, L.M. Pilarski, C.J. Backhouse| title = An integrated microfluidic chip for chromosome enumeration using fluorescence in situ hybridization| journal = Lab on a Chip| accessdate = 2009-03-24| date = 2008-11| url = http://www.rsc.org/publishing/journals/LC/article.asp?doi=b812443d}}</ref>, this technology will make wide-spread genetic testing of patients more accessible in a clinical setting. |
Compared to conventional FISH methods, these first implementations of on-chip FISH provide a 10-fold higher throughput and a 10-fold reduction in the cost of testing, enabling the simultaneous assessment of several chromosomal abnormalities or patients.<ref>{{Cite journal| doi = 10.1049/iet-nbt:20060021| volume = 1| issue = 3| pages = 27-35| last = Sieben| first = V.J.| coauthors = C.S. Debes Marun, P.M. Pilarski, G.V. Kaigala, L.M. Pilarski, C.J. Backhouse| title = FISH and chips: chromosomal analysis on microfluidic platforms| journal = IET Nanobiotechnology| accessdate = 2009-01-26| date = 2007-06| url = http://link.aip.org/link/?NBT/1/27/1}}</ref> It is increasingly essential that diagnostic tests determine the type and extent of chromosomal abnormalities for more informed diagnosis and for appropriate choice of treatment strategies. Since the on-chip FISH technique is 10-20 times more cost-effective than conventional methods, and can be fully integrated and automated<ref>{{Cite journal| doi = 10.1039/b812443d| volume = 8| issue = 12| pages = 2151-2156| last = Sieben| first = V.J.| coauthors = C.S. Debes-Marun, L.M. Pilarski, C.J. Backhouse| title = An integrated microfluidic chip for chromosome enumeration using fluorescence in situ hybridization| journal = Lab on a Chip| accessdate = 2009-03-24| date = 2008-11| url = http://www.rsc.org/publishing/journals/LC/article.asp?doi=b812443d}}</ref>, this technology will make wide-spread genetic testing of patients more accessible in a clinical setting. |
||
| Line 69: | Line 67: | ||
* [[Virtual Karyotype]] |
* [[Virtual Karyotype]] |
||
== Gallery == |
|||
<gallery> |
|||
| ⚫ | |||
| ⚫ | |||
</gallery> |
|||
==References== |
==References== |
||
{{reflist}} |
{{reflist}} |
||
Revision as of 20:06, 23 April 2009
This article needs additional citations for verification. (January 2009) |
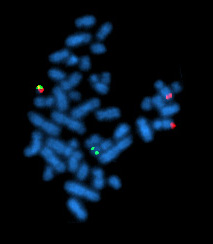
FISH (fluorescence in situ hybridization) is a cytogenetic technique used to detect and localize the presence or absence of specific DNA sequences on chromosomes. FISH uses fluorescent probes that bind to only those parts of the chromosome with which they show a high degree of sequence similarity. Fluorescence microscopy can be used to find out where the fluorescent probe bound to the chromosomes. FISH is often used for finding specific features in DNA for use in genetic counseling, medicine, and species identification. FISH can also be used to detect and localize specific mRNAs within tissue samples. In this context, it can help define the spatial-temporal patterns of gene expression within cells and tissues.
Probes

Probes are often derived from fragments of DNA that were isolated, purified, and amplified for use in the Human Genome Project. The size of the human genome is so large, compared to the length that could be sequenced directly, that it was necessary to divide the genome into fragments. The fragments were added into a framework that made it possible to use bacteria to replicate the fragments. The fragments were put into order by analyzing size-exclusion separation of enzymatically-digested fragments. Clonal populations of bacteria, each population maintaining a single artificial chromosome, are stored in various laboratories around the world. The artificial chromosomes (BAC) can be grown, extracted, and labeled, in any lab. These fragments are on the order of 100 thousand base-pairs, and are the basis for most FISH probes.
Preparation and Hybridization Process

First, a probe is constructed. The probe must be large enough to hybridize specifically with its target but not so large as to impede the hybridization process. The probe is tagged directly with fluorophores, with targets for antibodies or with biotin. Tagging can be done in various ways, such as nick translation, or PCR using tagged nucleotides.
Then, an interphase or metaphase chromosome preparation is produced. The chromosomes are firmly attached to a substrate, usually glass. Repetitive DNA sequences must be blocked by adding short fragments of DNA to the sample. The probe is then applied to the chromosome DNA and incubated for approximately 12 hours while hybridizing. Several wash steps remove all unhybridized or partially-hybridized probes. The results are then visualized and quantified using a microscope that is capable of exciting the dye and recording images.
If the fluorescent signal is weak, amplification of the signal may be necessary in order to exceed the detection threshold of the microscope. Fluorescent signal strength depends on many factors such as probe labeling efficiency, the type of probe, and the type of dye. Fluorescently-tagged antibodies or streptavidin are bound to the dye molecule. These secondary components are selected so that they have a strong signal.
FISH experiments designed to detect or localize gene expression within cells and tissues rely on the use of a reporter gene, such as one expressing green fluorescent protein, to provide the fluorescence signal.
Fiber FISH
In an alternative technique to interphase or metaphase preparations, fiber FISH, interphase chromosomes are attached to a slide in such a way that they are stretched out in a straight line, rather than being tightly coiled, as in conventional FISH, or adopting a random conformation, as in interphase FISH. This is accomplished by applying mechanical shear along the length of the slide, either to cells that have been fixed to the slide and then lysed, or to a solution of purified DNA. A technique known as chromosome combing is increasingly used for this purpose. The extended conformation of the chromosomes allows dramatically higher resolution - even down to a few kilobases. The preparation of fiber FISH samples, although conceptually simple, is a rather skilled art, and only specialized laboratories use the technique routinely.
Variations on probes and analysis

FISH is a very general technique. It is often arbitrarily divided into more specific categories based on application, but each category is similar in that, in a chemical sense, the technique is the same -- hybridization is the common denominator. The differences between the various FISH techniques are usually due to the construction and content of the fluorescently-labeled DNA probe. The size, overlap, colour, and mixture of the probes make possible all FISH techniques.
Probe size is important because longer probes hybridize more specifically than shorter probes. The overlap defines the resolution of detectable features. If the goal of an experiment is to detect the breakpoint of a translocation, then the overlap of the probes — the degree to which one DNA sequence is contained in the adjacent probes — defines the minimum window in which the breakpoint occurs.
The mixture of probes determines the type of feature the probe can detect. Probes that hybridize along an entire chromosome are used to count the number of a certain chromosome, show translocations, or identify extra-chromosomal fragments of chromatin. This is often called "whole-chromosome painting." If every possible probe is used, every chromosome, (in essence the whole genome) would be marked fluorescently, which would not be particularly useful for determining features of individual sequences. A mixture of smaller probes can be created that are specific to a particular region (locus) of DNA; these mixtures are used to detect deletion mutations. When combined with a specific colour, a locus-specific probe mixture is used to detect very specific translocations. Special locus-specific probe mixtures are often used to count chromosomes, by binding to the centromeric regions of chromosomes, which are unique enough to identify each chromosome (with the exception of Chromosome 13, 14 21, 22.)
Because modern microscopes can detect a range of colours in fluorescent dyes, each human chromosome can be identified (M-FISH) using whole-chromosome probe mixtures and a variety of colours. There are currently twice as many chromosomes as fluorescent dye colours. However, ratios of probe mixtures can be used to create additional colours. As with comparative genomic hybridization, the probe mixture for the secondary colours is created by mixing the correct ratio of two sets of differently-labeled probes for the same chromosome. Differently-coloured probes can be used for the detection of translocations. Several techniques exploit the resolution limitations of microscopes to resolve spatial distributions of dye below a few hundred nanometers. Colours that are adjacent appear to overlap, and a secondary colour is observed.
In reciprocal translocations, where both breakpoints are known, locus-specific probes are made for it and part of the region one either side of breakpoint. In normal cells, two colours will be visible; in diseased cells such as those found in BCR/ABL translocations, the two dye colours overlap, and a third colour is observed. This technique is known as double-fusion FISH or D-FISH. In translocations where only one of the breakpoints is known or constant, locus-specific probes are made for one side of the breakpoint and the other intact chromosome. In normal cells, the secondary colour is observed, but only the primary colour is observed when the translocation occurs. This technique is known as "break-apart FISH".
Medical applications
Often parents of children with a developmental delay want to know more about their child's conditions before choosing to have another child. These concerns can be addressed by analysis of the parents' and child's DNA. In cases where the child's developmental delay is not understood, the cause of it can be determined using FISH and cytogenetic techniques. Examples of diseases that are diagnosed using FISH include Prader-Willi syndrome, Angelman syndrome, 22q13 deletion syndrome, chronic myelogenous leukemia, acute lymphoblastic leukemia, Cri-du-chat, Velocardiofacial syndrome, and Down syndrome.
In medicine, FISH can be used to form a diagnosis, to evaluate prognosis, or to evaluate remission of a disease, such as cancer. Treatment can then be specifically tailored. A traditional exam involving metaphase chromosome analysis is often unable to identify features that distinguish one disease from another, due to subtle chromosomal features; FISH can elucidate these differences. FISH can also be used to detect diseased cells more easily than standard Cytogenetic methods, which require dividing cells and requires labor and time-intensive manual preparation and analysis of the slides by a technologist. FISH, on the other hand, does not require living cells and can be quantified automatically, a computer counts the fluorescent dots present. However, a trained technologist is required to distinguish subtle differences in banding patterns on bent and twisted metaphase chromosomes.
Species identification
FISH is often used in clinical studies. If a patient is infected with a suspected pathogen, bacteria, from the patient's tissues or fluids, are typically grown on agar to determine the identity of the pathogen. Many bacteria, however, even well-known species, do not grow well under laboratory conditions. FISH can be used to detect directly the presence of the suspect on small samples of patient's tissue.
FISH can also be to used compare the genomes of two biological species, to deduce evolutionary relationships. A similar hybridization technique is called a zoo blot. Bacterial FISH probes are often primers for the 16s rRNA region.
FISH is widely used in the field of microbial ecology, to identify microorganisms. Biofilms, for example, are composed of complex (often) multi-species bacterial organizations. Preparing DNA probes for one species and performing FISH with this probe allows one to visualize the distribution of this specific species within the biofilm. Preparing probes (in two different colors) for two species allows to visualize/study co-localization of these two species in the biofilm, and can be useful in determining the fine architecture of the biofilm.
Lab-on-a-chip and FISH

Although interphase fluorescence in situ hybridization (FISH) is a sensitive diagnostic tool used for the detection of chromosomal abnormalities on cell-by-cell basis, the cost-per-test and the technical complexity of current FISH protocols has inhibited its widespread utilization. Lab-on-a-chip or microfluidic devices, incorporate networks of microchannels that can miniaturize, integrate and automate conventional analytical techniques onto chip-style platforms. Since microchannels permit sophisticated levels of fluid control (down to picolitres), these devices can reduce analysis times, lower reagent consumption, and minimize human intervention.
Currently, FISH has been performed on glass microfluidic platforms that standardize much of the protocol offering repeatable results that are accurate, cost-effective and easier to obtain in a clinical setting.
Compared to conventional FISH methods, these first implementations of on-chip FISH provide a 10-fold higher throughput and a 10-fold reduction in the cost of testing, enabling the simultaneous assessment of several chromosomal abnormalities or patients.[1] It is increasingly essential that diagnostic tests determine the type and extent of chromosomal abnormalities for more informed diagnosis and for appropriate choice of treatment strategies. Since the on-chip FISH technique is 10-20 times more cost-effective than conventional methods, and can be fully integrated and automated[2], this technology will make wide-spread genetic testing of patients more accessible in a clinical setting.
Virtual karyotyping is another cost-effective, clinically available alternative to FISH panels uses thousands to millions of probes on a single array to detect copy number changes, genome-wide, at unprecedented resolution.
See also
Gallery
-
Another schematic of FISH process.
-
Microfluidic chip that lowered the cost-per-test of FISH by 90%.
References
- ^ Sieben, V.J. (2007-06). "FISH and chips: chromosomal analysis on microfluidic platforms". IET Nanobiotechnology. 1 (3): 27–35. doi:10.1049/iet-nbt:20060021. Retrieved 2009-01-26.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sieben, V.J. (2008-11). "An integrated microfluidic chip for chromosome enumeration using fluorescence in situ hybridization". Lab on a Chip. 8 (12): 2151–2156. doi:10.1039/b812443d. Retrieved 2009-03-24.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)
- Annelie Pernthaler, Jakob Pernthaler, Rudolf Amann (2002): Fluorescence In Situ Hybridization and Catalyzed Reporter Deposition
for the Identification of Marine Bacteria, APPLIED AND ENVIRONMENTAL MICROBIOLOGY, June 2002, p. 3094–3101 Vol. 68, No. 6 DOI: 10.1128/AEM.68.6.3094–3101.2002
- Michael Wagner, Matthias Horny and Holger Daimsz (2003): Fluorescence in situ hybridisation for the identification and
characterisation of prokaryotes. Current Opinion in Microbiology 2003, 6:302–309 DOI:10.1016/S1369-5274(03)00054-7
External links
- Fluorescence+in+Situ+Hybridization at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Information on fiber FISH from the Olympus Corporation
- A guide to fiber FISH from Octavian Henegariu
- Fibre FISH protocol from the Human Genome Project at the Sanger Centre
- CARD-FISH, BioMineWiki
- Preparation of Complex DNA Probe Sets for 3D FISH with up to Six Different Fluorochromes
- FISH technical notes and protocols from GeneDetect.com
- Fluorescence in situ Hybridization Photos of bacteria


