Uranium hexafluoride: Difference between revisions
No edit summary |
|||
| Line 64: | Line 64: | ||
[[Image:DUF6 storage yard far.jpg|255px|left]] |
[[Image:DUF6 storage yard far.jpg|255px|left]] |
||
About 95% of the [[depleted uranium]] produced to date is stored as uranium hexafluoride, DUF<sub>6</sub>, in steel cylinders in open air yards close to enrichment plants. Each cylinder contains up to 12.7 tonnes (or 14 US tons) of solid UF<sub>6</sub>. The long-term storage of DUF<sub>6</sub> presents environmental, health, and safety risks because of its chemical instability. When UF<sub>6</sub> is exposed to moist air, it reacts with the water in the air to produce UO<sub>2</sub>F<sub>2</sub> ([[uranyl fluoride]]) and HF ([[hydrogen fluoride]]) both of which are highly soluble and toxic. Storage cylinders must be regularly inspected for signs of corrosion and leaks. The estimated life time of the steel cylinders is measured in decades.<ref>{{cite web | publisher = Institute for Energy and Environmental Research | date = December 1997 | title = What is DUF<sub>6</sub>? Is it dangerous and what should we do with it? | url = http://www.ieer.org/sdafiles/vol_5/5-2/deararj.html | date = 2007-09-24}}</ref> |
About 95% of the [[depleted uranium]] produced to date is stored as uranium hexafluoride, DUF<sub>6</sub>, in steel cylinders in open air yards close to enrichment plants. Each cylinder contains up to 12.7 tonnes (or 14 US tons) of solid UF<sub>6</sub>. In the U.S. alone, 560,000 tonnes of depleted UF<sub>6</sub> had accumulated by 1993. In 2005, 686,500 tonnes in 57,122 storage cylinders were located near Portsmouth, Ohio, Oak Ridge, Tennessee, and Paducah, Kentucky.<ref>{{cite web | work = Depleted UF<sub>6</sub> FAQs | title = How much depleted uranium hexafluoride is stored in the United States? | url = http://web.ead.anl.gov/uranium/faq/health/faq16.cfm | publisher = [[Argonne National Laboratory]]}}</ref><ref>[http://web.ead.anl.gov/uranium/documents/index.cfm Documents<!-- Bot generated title -->]</ref> The long-term storage of DUF<sub>6</sub> presents environmental, health, and safety risks because of its chemical instability. When UF<sub>6</sub> is exposed to moist air, it reacts with the water in the air to produce UO<sub>2</sub>F<sub>2</sub> ([[uranyl fluoride]]) and HF ([[hydrogen fluoride]]) both of which are highly soluble and toxic. Storage cylinders must be regularly inspected for signs of corrosion and leaks. The estimated life time of the steel cylinders is measured in decades.<ref>{{cite web | publisher = Institute for Energy and Environmental Research | date = December 1997 | title = What is DUF<sub>6</sub>? Is it dangerous and what should we do with it? | url = http://www.ieer.org/sdafiles/vol_5/5-2/deararj.html | date = 2007-09-24}}</ref> |
||
[[Image:DUF6 cylinder leak.gif|255px|right]] |
[[Image:DUF6 cylinder leak.gif|255px|right]] |
||
Revision as of 22:20, 9 May 2009

| |
| Names | |
|---|---|
| IUPAC names
Uranium hexafluoride
Uranium(VI) fluoride | |
| Identifiers | |
| ECHA InfoCard | 100.029.116 |
| RTECS number |
|
| UN number | 2978 (<1% 235U) 2977 (>1% 235U) |
CompTox Dashboard (EPA)
|
|
| Properties | |
| UF6 | |
| Molar mass | 352.02 g/mol |
| Appearance | colorless solid |
| Density | 5.09 g/cm3, solid |
| Melting point | 64.8 °C (338.0 K) |
| Boiling point | 56.5 °C (329.7 K) (sublimes) |
| Decomposes | |
| Structure | |
| Octahedral | |
| zero | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
228 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−2317 kJ/mol |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Uranium hexachloride |
Other cations
|
Neptunium hexafluoride Plutonium hexafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Uranium hexafluoride (UF6), referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure (STP), is highly toxic, reacts violently with water and is corrosive to most metals. It reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists further reaction.
Milled uranium ore — U3O8, or "yellowcake" — is dissolved in nitric acid, yielding a solution of uranyl nitrate UO2(NO3)2. Pure uranyl nitrate is obtained by solvent extraction, then treated with ammonia to produce ammonium diuranate ("ADU", (NH4)2U2O7). Reduction with hydrogen gives UO2, which is converted with hydrofluoric acid (HF) to uranium tetrafluoride, UF4. Oxidation with fluorine finally yields UF6.

Application in the nuclear fuel cycle
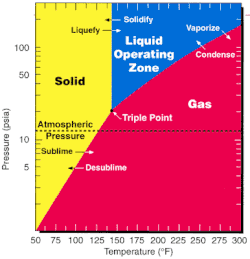
UF6 is used in both of the main uranium enrichment methods, gaseous diffusion and the gas centrifuge method, because it has a triple point at 147 °F (64 °C, 337 K) and slightly higher than normal atmospheric pressure. Additionally, fluorine has only a single stable naturally occurring isotope, so isotopologues of UF6 differ in their molecular weight based solely on the uranium isotope present.[1]
All the other uranium fluorides are involatile solids which are coordination polymers.
Gaseous diffusion requires ca. 60 times as much energy as the gas centrifuge process; even so, this is just 4% of the energy that can be produced by the resulting enriched uranium.
In addition to its use in enrichment, uranium hexafluoride has been used in an advanced reprocessing method (fluoride volatility) which was developed in the Czech Republic. In this process, used oxide nuclear fuel is treated with fluorine gas to form a mixture of fluorides. This is then distilled to separate the different classes of material.
Storage in gas cylinders

About 95% of the depleted uranium produced to date is stored as uranium hexafluoride, DUF6, in steel cylinders in open air yards close to enrichment plants. Each cylinder contains up to 12.7 tonnes (or 14 US tons) of solid UF6. In the U.S. alone, 560,000 tonnes of depleted UF6 had accumulated by 1993. In 2005, 686,500 tonnes in 57,122 storage cylinders were located near Portsmouth, Ohio, Oak Ridge, Tennessee, and Paducah, Kentucky.[2][3] The long-term storage of DUF6 presents environmental, health, and safety risks because of its chemical instability. When UF6 is exposed to moist air, it reacts with the water in the air to produce UO2F2 (uranyl fluoride) and HF (hydrogen fluoride) both of which are highly soluble and toxic. Storage cylinders must be regularly inspected for signs of corrosion and leaks. The estimated life time of the steel cylinders is measured in decades.[4]

There have been several accidents involving uranium hexafluoride in the United States.[5][6] The U.S. government has been converting DUF6 to solid uranium oxides for disposal.[7] Such disposal of the entire DUF6 inventory could cost anywhere from $15 million to $450 million.[8]
Chemistry
The solid state structure was reported by J.H. Levy, J.C Taylor and A.B Waugh.[9] In this paper neutron diffraction was used to determine the structures of UF6, MoF6 and WF6 at 77K.
-
This is a simple mononuclear molecule
-
The crystal structure of uranium hexafluoride
It has been shown that uranium hexafluoride is an oxidant and a lewis acid which is able to bind to fluoride, for instance the reaction of copper fluoride with uranium hexafluoride in acetonitrile is reported to form Cu[UF7]2.5MeCN.[10]
Polymeric uranium(VI) fluorides containing organic cations have been isolated and characterised by X-ray diffraction.[11]
At room pressure, it sublimes at 56.5 °C.[12] The triple point is at 64 °C.[13]
Other uranium fluorides
The pentafluoride of uranium (UF5) and diuranium nonafluoride (U2F9) has been characterised by C.J. Howard, J.C Taylor and A.B. Waugh.[14]
The trifluoride of uranium was characterised by J. Laveissiere.[15] The structure of UOF4 was reported by J.H. Levy, J.C. Taylor, and P.W. Wilson.[16]
See also
References
- ^ "Uranium Enrichment and the Gaseous Diffusion Process". USEC Inc. Retrieved 2007-09-24.
- ^ "How much depleted uranium hexafluoride is stored in the United States?". Depleted UF6 FAQs. Argonne National Laboratory.
- ^ Documents
- ^ "What is DUF6? Is it dangerous and what should we do with it?". Institute for Energy and Environmental Research. 2007-09-24.
- ^ "Have there been accidents involving uranium hexafluoride?". Depleted UF6 FAQs. Argonne National Laboratory.
- ^ "Uranium Hexafluoride (UF6) Tailings: Characteristics, Transport and Storage at the Siberian Chemical Combine (Sibkhimkombinat) Tomsk" (briefing note). Large and Associates. 5 November 2005. Retrieved 2007-09-24.
{{cite web}}: Check date values in:|date=(help) - ^ "What is going to happen to the uranium hexafluoride stored in the United States?". Depleted UF6 FAQs. Argonne National Laboratory.
- ^ "Are there any currently-operating disposal facilities that can accept all of the depleted uranium oxide that would be generated from conversion of DOE's depleted UF6 inventory?". Depleted UF6 FAQs. Argonne National Laboratory.
- ^ J.H. Levy, J.C Taylor and A.B Waugh (1983). "Neutron powder structural studies of UF6, MoF6 and WF6 at 77 K". Journal of Fluorine Chemistry. 23: 29–36. doi:10.1016/S0022-1139(00)81276-2.
- ^ Berry JA, Poole RT, Prescott A, Sharp DWA, Winfield JM (1976). "The oxidising and fluoride ion acceptor properties of uranium hexafluoride in acetonitrile". J. Chem. Soc. Dalton Trans.: 272. doi:10.1039/DT9760000272.
{{cite journal}}: CS1 maint: multiple names: authors list (link) x - ^ Walker SM, Halasyamani PS, Allen S, O'Hare D (1999). "From Molecules to Frameworks: Variable Dimensionality in the UO2(CH3COO)2·2H2O/HF(aq)/Piperazine System. Syntheses, Structures, and Characterization of Zero-Dimensional (C4N2H12)UO2F4·3H2O, One-Dimensional (C4N2H12)2U2F12·H2O, Two-Dimensional (C4N2H12)2(U2O4F5)4·11H2O, and Three-Dimensional (C4N2H12)U2O4F6". J. Am. Chem. Soc. 121: 10513. doi:10.1021/ja992145f.
{{cite journal}}: CS1 maint: multiple names: authors list (link) x - ^ http://nuclearweaponarchive.org/Library/Glossary
- ^ Uranium Hexafluoride: Source: Appendix A of the PEIS (DOE/EIS-0269): Physical Properties
- ^ Howard CJ, Taylor JC, Waugh AB (1982). "Crystallographic parameters in α-UF5 and U2F9 by multiphase refinement of high-resolution neutron powder data". Journal of Solid State Chemistry. 45: 396–398. doi:10.1016/0022-4596(82)90185-2.
{{cite journal}}: CS1 maint: multiple names: authors list (link) x - ^ Laveissiere J (1967). Bulletin de la Societe Francaise de Mineralogie et de Cristallographie. 90: 304–307.
{{cite journal}}: Missing or empty|title=(help) - ^ Levy JH, Taylor JC, Wilson PW (1977). "Structure of fluorides .17. NEUTRON-DIFFRACTION STUDY OF ALPHA-URANIUM OXIDE TETRAFLUORIDE". Journal of Inorganic and Nuclear Chemistry. 39: 1989–1991. doi:10.1016/0022-1902(77)80531-9.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- Levy JH (1976). "Structure of fluorides. Part XII. Single-crystal neutron diffraction study of uranium hexafluoride at 293 K". J. Chem. Soc. Dalton Trans.: 219. doi:10.1039/DT9760000219.x (xstal structure)
- Olah GH, Welch J (1978). "Synthetic methods and reactions. 46. Oxidation of organic compounds with uranium hexafluoride in haloalkane solutions". J. Am. Chem. Soc. 100: 5396. doi:10.1021/ja00485a024. x (selective oxidant of CFCs)






