Biopolymer: Difference between revisions
| Line 265: | Line 265: | ||
Since the introduction of plastic films in the 1930s and 1940s for greenhouse coverings, fumigation and mulching, agricultural applications of polymers have grown at an enormous rate. All principal classes of polymers, i.e. plastics, coatings, elastomers, fibres and water-soluble polymers are presently utilized in applications which include the controlled release of pesticides and nutrients, soil conditioning, seed coatings, gel plantings and plant protection. However, degradable plastics are also of interest as agricultural mulches and agricultural planting containers. Ultimate biodegradability, as in composting, is also of some interest as it would permit degradable plastics to be combined with other biodegradable materials and converted into useful soil-improving materials. |
Since the introduction of plastic films in the 1930s and 1940s for greenhouse coverings, fumigation and mulching, agricultural applications of polymers have grown at an enormous rate. All principal classes of polymers, i.e. plastics, coatings, elastomers, fibres and water-soluble polymers are presently utilized in applications which include the controlled release of pesticides and nutrients, soil conditioning, seed coatings, gel plantings and plant protection. However, degradable plastics are also of interest as agricultural mulches and agricultural planting containers. Ultimate biodegradability, as in composting, is also of some interest as it would permit degradable plastics to be combined with other biodegradable materials and converted into useful soil-improving materials. |
||
==== |
==== Mulching films ==== |
||
Mulches permit growers to use plastic films to help with plant growth and then photodegrade in the fields thereby avoiding the cost of removal. The plastic films are desirable because they conserve moisture, reduce weeds and increase soil temperatures, thus improving the rate of growth in plants. For example, a 6 ha melon farm reported a two- to three-fold increase in yield and ripening two weeks earlier as a result of using black polyethylene mulch. Elimination of weeds and avoidance of soil compaction by the use of mulch eliminates the need for cultivation, therefore root damage and stunting or killing of plants is further avoided. Fertilizer and water requirements are also reduced. |
Mulches permit growers to use plastic films to help with plant growth and then photodegrade in the fields thereby avoiding the cost of removal. The plastic films are desirable because they conserve moisture, reduce weeds and increase soil temperatures, thus improving the rate of growth in plants. For example, a 6 ha melon farm reported a two- to three-fold increase in yield and ripening two weeks earlier as a result of using black polyethylene mulch. Elimination of weeds and avoidance of soil compaction by the use of mulch eliminates the need for cultivation, therefore root damage and stunting or killing of plants is further avoided. Fertilizer and water requirements are also reduced. |
||
Researchers found that a starch–polyvinyl alcohol film could be coated with a thin layer of water-resistant polymer to give a degradable agricultural mulching film. Starch-based polyethylene films were formulated and consisted of up to 40% starch, urea, ammonia and various portions of low-density polyethylene (LDPE) and poly(ethylene-co-acrylic acid) (EAA). The EAA acted as a compatibilizer, forming a complex between the starch and the PE in the presence of ammonia. The resulting blend could be cast or blown into films, and had physical properties approaching those of LDPE. |
|||
| ⚫ | |||
In one trial, three techniques were used to incorporate large amounts of starch as a filler into disposable polyvinyl chloride (PVC) plastics. In the first technique, a starch xanthate solution was prepared by mixing starch with aqueous NaOH and then adding a small amount of carbon disulphide (usually 0.1 mol CS<sub>2</sub> per mol starch). To this starch–xanthate solution, a PVC latex was added. The starch–xanthate and PVC resins were then co-precipitated by adding NaNO<sub>2</sub> and alum. The fine powder obtained from this was blended with dioctyl phthalate (DOP). In the second technique (a concentration method), whole starch was gelatinized by heating in water before mixing into the PVC latex. After removing the water, dry product was mixed with DOP. In the third method, starch was dry-blended with PVC and DOP. These films appear to be useful for a variety of agricultural applications. |
|||
In addition, transparent polyethylene is more effective in trapping heat than black or smoke-grey films. Soil temperatures may rise 5.5°C under clear films, as compared to 1.7 – 2.7°C under black films. Radiative heat loss at night, as the soil cools, is lessened by polymer films. In some cases, weed control has been reported because of solar heating of the polyethylene mulches. |
|||
| ⚫ | If left in place, however, conventional films can cause problems during harvesting or during cultivating operations the next year. Removal and disposal are costly and inconvenient. Therefore, interest in the development of biodegradable or photodegradable films with short service lifetimes has grown. Although a large number of polymer types could be designed for controlled degradation, only a few have been commercialized. Plastics used for this purpose usually contain light-sensitizing additives which cause the materials to undergo photodegradation. |
||
The plastics used for mulch films are generally low density polyethylenes, poly(vinyl chloride), polybutylene or copolymers of ethylene with vinyl acetate. A particularly interesting photodegradable system consists of a mixture of ferric and nickel dibutyldithiocarbamates, the ratio of which is adjusted to provide protection for specific growing periods. The degradation is tuned so that when the growing season is over the plastic will begin to photodegrade. Another additive system being proposed for this application includes a combination of substituted benzophenones and titanium or zirconium chelates. The principal commercial degradable mulch is photodegradable poly(1-butene). |
The plastics used for mulch films are generally low density polyethylenes, poly(vinyl chloride), polybutylene or copolymers of ethylene with vinyl acetate. A particularly interesting photodegradable system consists of a mixture of ferric and nickel dibutyldithiocarbamates, the ratio of which is adjusted to provide protection for specific growing periods. The degradation is tuned so that when the growing season is over the plastic will begin to photodegrade. Another additive system being proposed for this application includes a combination of substituted benzophenones and titanium or zirconium chelates. The principal commercial degradable mulch is photodegradable poly(1-butene). |
||
Revision as of 03:26, 12 July 2010

Biopolymers are polymers produced by living organisms. Cellulose, starch and chitin, proteins and peptides, and DNA and RNA are all examples of biopolymers, in which the monomeric units, respectively, are sugars, amino acids, and nucleotides. [1] [2] [3] [4]
Cellulose is both the most common biopolymer and the most common organic compound on Earth. About 33 percent of all plant matter is cellulose. E.G. The cellulose content of cotton is ~ 90 percent and that of wood is ~ 50 percent. [5]
Some biopolymers are biodegradable. That is, they are broken down into CO2 and water by microorganisms. In addition, some of these biodegradable biopolymers are compostable. That is, they can be put into an industrial composting process and will break down by 90% within 6 months. Biopolymers that do this can be marked with a 'compostable' symbol, under European Standard EN 13432 (2000). Packaging marked with this symbol can be put into industrial composting processes and will break down within 6 months (or less). An example of a compostable polymer is PLA film under 20 μm thick: films which are thicker than that do not qualify as compostable, even though they are biodegradable. A home composting logo may soon be established which will enable consumers to dispose of packaging directly onto their own compost heap. [6]
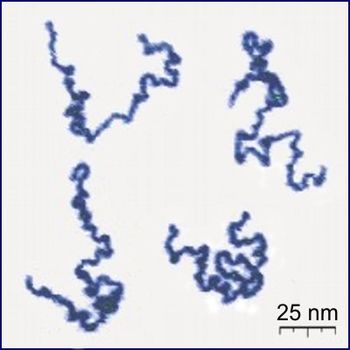
Introduction
A major but defining difference between polymers and biopolymers can be found in their structures. Polymers, including biopolymers, are made of repetitive units called monomers. Biopolymers often have a well defined structure, though this is not a defining characteristic (example:ligno-cellulose): The exact chemical composition and the sequence in which these units are arranged is called the primary structure, in the case of proteins. Many biopolymers spontaneously fold into characteristic compact shapes (see also "protein folding" as well as secondary structure and tertiary structure), which determine their biological functions and depend in a complicated way on their primary structures. Structural biology is the study of the structural properties of the biopolymers. In contrast most synthetic polymers have much simpler and more random (or stochastic) structures. This fact leads to a molecular mass distribution that is missing in biopolymers. In fact, as their synthesis is controlled by a template directed process in most in vivo systems all biopolymers of a type (say one specific protein) are all alike: they all contain the similar sequences and numbers of monomers and thus all have the same mass. This phenomenon is called monodispersity in contrast to the polydispersity encountered in synthetic polymers. As a result biopolymers have a polydispersity index of 1. [8]
Overview
Biopolymers (also called renewable polymers) are generally produced from biomass, which comes from crops such as sugar beet, potatoes or wheat: when used to produce biopolymers, these are classified as non food crops. These can be converted in the following pathways:
Sugar beet > Glyconic acid > Polyglonic acid
Starch > (fermentation) > Lactic acid > Polylactic acid (PLA)
Biomass > (fermentation) > Bioethanol > Ethene > Polyethylene
Biopolymers are renewable, sustainable, and can be carbon neutral
Biopolymers are renewable, because they are made from plant materials which can be grown year on year indefinitely. These plant materials come from agricultural non food crops. Therefore, the use of biopolymers would create a sustainable industry. In contrast, the feedstocks for polymers derived from petrochemicals will eventually run out. In addition, biopolymers have the potential to cut carbon emissions and reduce CO2 quantities in the atmosphere: this is because the CO2 released when they degrade can be reabsorbed by crops grown to replace them: this makes them close to carbon neutral.
Biopolymers are biodegradable, and some are also compostable
Some biopolymers are biodegradable: they are broken down into CO2 and water by microorganisms. In addition, some of these biodegradable biopolymers are compostable: they can be put into an industrial composting process and will break down by 90% within 6 months. Biopolymers that do this can be marked with a 'compostable' symbol, under European Standard EN 13432 (2000).
Polysaccharides
Sugar-based biopolymers are often difficult with regards to convention. Sugar polymers can be linear or branched are typically joined with glycosidic bonds. However, the exact placement of the linkage can vary and the orientation of the linking functional groups is also important, resulting in α- and β-glycosidic bonds with numbering definitive of the linking carbons' location in the ring. In addition, many saccharide units can undergo various chemical modification, such as amination, and can even form parts of other molecules, such as glycoproteins.
For materials applications, the polysaccharides currently receiving the most attention for industrial applications are cellulose and starch. But increasing attention is being given to the more complex carbohydrate polymers which are produced by bacteria and fungi. Examples include xanthan, curdlan, pullulan and hyaluronic acid. These latter polymers generally contain more than one type of carbohydrate monomer, and in many cases these polymers have ordered branched structures. Starch, for example, is a physical combination of branched and linear polymers (amylopectin and amylose, respectively), but it contains only a single type of carbohydrate (or sugar): glucose. Both cellulose and starch are composed of hundreds or thousands of glucopyranoside repeating units. These units are linked together by acetal bonds formed between the carbon atom of the cyclic glucose in one unit and a hydroxyl group in the adjacent unit.
Starch

Starch is a polymer which occurs widely in plants. The principal crops used for its production include potatoes, corn and rice. In all of these plants, starch is produced in the form of granules, which vary in size and composition from plant to plant. In general, the linear polymer, amylose, makes up about 20 wt% of the granule, and the branched polymer, amylopectin, the remainder. Amylose is crystalline and can have a number average molecular weight as high as 500,000, but it is soluble in boiling water. Amylopectin is insoluble in boiling water, but in their use in foods, both fractions are readily hydrolyzed at the acetal link by enzymes.
Starch has been widely used as a raw material in film production because of increasing prices and decreasing availability of conventional film-forming resins. Starch films possess low permeability and are thus attractive materials for food packaging. Starch is also useful for making agricultural mulch films because it degrades into harmless products when placed in contact with soil microorganisms.
Research on starch includes investigation of its water adsorption, chemical modification, and its resistance to thermomechanical shear. Although starch is a polymer, its stability under stress is not high. At temperatures higher than 150°C, the glucoside links start to break, and above 250°C the starch grain endothermally collapses. At low temperatures, a phenomenon known as retrogradation is observed. This is a reorganization of the hydrogen bonds and an aligning of the molecular chains during cooling. In extreme cases under 10°C, precipitation is observed. Thus, though starch can be dispersed into hot water and cast as films, the above phenomenon yields to a plastic-brittle transition and ultimalte brittle failure in the film.
In its application in biodegradable plastics, starch is either physically mixed in with its native granules, kept intact, or melted and blended on a molecular level with the appropriate polymer. In either form, the fraction of starch in the mixture which is accessible to enzymes can be degraded by either, or both, amylases and glucosidases.
The starch molecule has two important functional groups, the –OH group that is susceptible to substitution reactions and the C–O–C bond that is susceptible to chain breakage. The hydroxyl group of glucose has a nucleophilic character. By reaction of its –OH group, modification of various properties can be obtained. One example is the reaction with silane to improve its dispersion in polyethylene. Crosslinking or bridging of the –OH groups changes the structure into a network while increasing the viscosity, reducing water retention and increasing its resistance to thermomechanical shear.

Acetylated starch does have several advantages as a structural fibre or film-forming polymer as compared to native starch. The acetylation of starch is a well-known reaction and is a relatively easy derivative to synthesize. Starch acetate is considerably more hydrophobic than is starch and has been shown to have better retention of tensile properties in aqueous environments. Another advantage is that starch acetate has an improved solubility compared to starch and is easily cast into films from simple solvents. The degree of acetylation is easily controlled by transesterification, allowing polymers to be produced with a range of hydrophobicities.
Starch has been acetylated [with a high content (70%) of linear amylose] and its enzymatic degradation studied. Starch acetate was prepared by acetylation of starch with a pyridine/acetic anhydride mixture and cast into films from solutions of 90% formic acid. A series of films with a range of acetyl content were then exposed to buffered amylase solutions. It was found that with sufficient acetyl content, the wet strength of the films was maintained in the aqueous solutions, but that the acetyl content was sufficiently low to permit degradation by a mixture of alpha and beta amylases within a period of 1 h. These films might be useful as membranes in bioreactors which could then be degraded by the addition of enzymes to the system.
Cellulose
Many polymer researchers believe that polymer chemistry had its origins with the characterization of cellulose. Cellulose was isolated for the first time some 150 years ago. Cellulose differs in some respects from other polysaccharides produced by plants, the molecular chain being very long and consisting of one repeating unit (cellobiose). Naturally, it occurs in a crystalline state. From the cell walls, cellulose is isolated in microfibrils by chemical extraction. In all forms, cellulose is a very highly crystalline, high molecular weight polymer, which is infusible and insoluble in all but the most aggressive, hydrogen bond-breaking solvents. Because of its infusibility and insolubility, cellulose is usually converted into derivatives to make it more user friendly in processing.
Some fungi can secrete enzymes that catalyze oxidation reactions of either cellulose itself or the lower molecular weight oligomers produced from the enzymatic hydrolysis of cellulose. Of these, the peroxidases can provide hydrogen peroxide for free radical attack on the C2–C3 positions of cellulose to form ‘aldehyde' cellulose, which is very reactive and can hydrolyze to form lower molecular weight fragments. Bacteria also secrete enzymes, some of which form complexes that act jointly in degrading cellulose to form carbohydrate nutrients which the microorganisms utilize for survival.
Aerobic soil environments generally contain a wide range of several different type of degrading bacteria and fungi which operate cooperatively. Primary microorganisms degrade cellulose to glucose and cellodextrins, a portion of which they utilize, and secondary microorganisms, which provide enzymes that degrade the cellodextrins to glucose, which they consume. By consuming glucose the latter assist in the growth of the primary microorganism because they prevent the build-up of the cellodextrins, which can inhibit glucanases if they are present in the environment at high concentrations. The final products from aerobic biodegradation are ultimately CO2 and water.
In anaerobic environments, a variety of final products are formed, including CO2, hydrogen, methane, hydrogen sulphide and ammonia. CO2 can be formed by oxidative reactions which utilize inorganic compounds, such as sulphate and nitrate ions, in the environment as oxidizing agents. Hydrogen produced by some anaerobic bacteria can be utilized by autotrophic bacteria to reduce oxidized compounds and CO2 to form either acetic acid or methane.
Cellulose has received more attention than any other polymer since it is attacked by a wide variety of microorganisms, and since it is often used in textiles without additives to complicate the interpretation of results. Cellulose represents an appreciable fraction of the waste products that make up sewage and refuse. It is fortunate that it does decompose readily. Fermentation of cellulose has been suggested as a source of chemicals such as ethanol and acetic acid, but this has not achieved any commercial importance to date.
All of the important derivatives of cellulose are reaction products of one or more of the three hydroxyl groups, including: (1) ethers, e.g. methyl cellulose and hydroxyl-ethyl cellulose; (2) esters, e.g. cellulose acetate and cellulose xanthate, which is used as a soluble intermediate for processing cellulose into either fibre or film forms, during which the cellulose is regenerated by controlled hydrolysis; and (3) acetals, especially the cyclic acetal formed between the C2 and C3 hydroxyl groups and butyraldehyde.
The biodegradation of cellulose is complicated, because cellulose exists together with lignin, for example, in wood cell walls. White-rot fungi secrete exocellular peroxidases to degrade lignin preferentially and, to a lesser extent, cellulases to degrade the polysaccharides in order to produce simple sugars which serve as nutrients for these microorganisms. Brown-rot fungi secrete enzymes for the degradation of cellulose and the hemicelluloses. Soft-rot fungi, also degrade principally these two types of polysaccharides.
Cellulose esters represent a class of polymers that have the potential to participate in the carbon cycle via microbiologically catalyzed de-esterification and decomposition of the resulting cellulose and organic acids. Cellulose acetate is currently used in high volume applications ranging from fibres, to films, to injection moulding thermoplastics. It has the physical properties and relatively low material costs that have tended to exclude other biodegradable polymers from being widely accepted in the marketplace.
Scientists have developed a series of cellulose acetate films, differing in their degree of substitution, that were evaluated in this bench-scale system. In addition, commercially available biodegradable polymers such as poly(hydroxybutyrate-co-valerate) (PHBV) and polycaprolactone (PCL) were included as points of reference. Based on film disintegration and film weight loss, cellulose acetates, having degrees of substitution less than approximately 2.20, compost at rates comparable to that of PHBV. NMR and GPC analyses of composted films indicate that low molecular weight fractions are removed preferentially from the more highly substituted and slower degrading cellulose acetates.
There has been evidence of esterase activity on soluble cellulose acetates with a low degree of substitution. A pure culture of Pestalotiopsis Westerdijkii Quarter Master (QM) 381 was reported to completely utilize this low DS cellulose ester. However, Reese did not find any evidence that the fully substituted cellulose triacetate could be biodegraded. Others have found evidence of esterase activity on reverse osmosis membranes composed of cellulose acetate.
The high level of microbial utilization of carbon from both cellulose esters and its conversion to CO2 confirms the biodegradability of these polymers and the potential they have for total mineralization in natural, microbiologically active environments. The biodegradation of cellulose ethers has been studied extensively and it is known that cellulose ethers with a DS of less than 1 will degrade due to attack of microorganisms at the unsubstituted residues of the polymers. The ether linkages on the cellulose backbone are considered resistant to microbial attack. By contrast, there have been conflicting reports concerning the biodegradation potential of cellulose esters.
Chitin and chitosan
Chitin is a macromolecule found in the shells of crabs, lobsters, shrimps and insects. Chitin can be degraded by chitinase. Chitin fibres have been utilized for making artificial skin and absorbable sutures. Chitin is insoluble in its native form but chitosan, the partly deacetylated form, is water soluble. The materials are biocompatible and have antimicrobial activities as well as the ability to absorb heavy metal ions. They also find applications in the cosmetic industry because of their water-retaining and moisturizing properties. Using chitin and chitosan as carriers, a water-soluble prodrug has been synthesized. Modified chitosans have been prepared with various chemical and biological properties. E.G. N-Carboxymethylchitosan and N-carboxybutylchitosan have been prepared for use in cosmetics and in wound treatment.
Chitin derivatives can also be used as drug carriers, and a report of the use of chitin in absorbable sutures shows that chitins have the lowest elongation among suture materials consisting of chitin, poly(glycolic acid) (PGA), plain catgut and chromic catgut. The tissue reaction of chitin is similar to that of PGA.
Alginic acid
Many polysaccharides in solution form gels upon the introduction of counterions. The degree of cross-linking is dependent on various factors such as pH, type of counterion, and the functional charge density of these polymers. Alginates have been studied extensively for their ability to form gels in the presence of divalent cations. Alginic acid forms water-soluble salts with monovalent cations, low molecular weight amines, and quaternary ammonium compounds. It becomes water-insoluble in the presence of polyvalent cations such as Ca2+, Be2+, Cu2+, Al3+ and Fe3+. Alginate gels have been used widely in controlled release drug delivery systems. Alginates have been used to encapsulate various herbicides, microorganisms and cells.
Polypeptides

The convention for a polypeptide is to list its constituent amino acid residues as they occur from the amino terminus to the carboxylic acid terminus. The amino acid residues are always joined by peptide bonds. Protein, though used colloquially to refer to any polypeptide, refers to larger or fully functional forms and can consist of several polypeptide chains as well as single chains. Proteins can also be modified to include non-peptide components, such as saccharide chains and lipids.
The proteins that have been the most widely used in industrial applications are generally neither soluble nor fusible without degradation. Thus they are used primarily in their natural form. This is especially true for the fibrous proteins wool, silk and collagen.
All proteins are specific co-polymers with regular arrangements of different types of α-amino acids. So the biosynthesis of proteins is an extremely complex process involving many different types of enzymes. In contrast, the enzymatic degradation of proteins, with general purpose proteases, is a relatively straightforward, amide hydrolysis reaction.
Gelatin
Gelatin is an animal protein which consists of 19 amino acids joined by peptide linkages and can be hydrolyzed by a variety of enzymes to yield its constituent amino acids or peptide components. This property makes gelatin desirable for purposeful biodegradation. Gelatin is a water-soluble, biodegradable polymer with extensive industrial, pharmaceutical, and biomedical uses, has been employed for coatings and microencapsulating various drugs, as well as for preparing biodegradable hydrogels.
A method was developed to prepare a simple, flexible gelatin film-based artificial skin that could adhere to an open wound and protect it against fluid loss and infection. The approach used was to mix polyglycerols with commercially available gelatin and to cast films on teflon-covered trays. The films were relatively tough, as well as being adherent to open wounds. They could be enriched with bioactive molecules, such as growth factors and antibiotics that would be released over several days. The films could be sterilized with γ-rays or prepared under sterile conditions.
Chemical modification of natural polymers by grafting serves the dual purpose of utilizing renewable, naturally derived products (e.g. proteins) as replacements for petroleum-based polymers and as biodegradable compositions which can be tailored for the slower or faster rates of degradation.
In order to extend the application of grafting for the modification of natural polymers, one group grafted methyl methacrylate onto gelatins by radical initiators and studied these in aqueous solution at temperatures between 60 and 80°C. From kinetic data, it was shown that: (1) the efficiency of grafting is higher at lower temperatures; (2) a sharp increase in the efficiency of grafting occurs at the later period of the polymerization at high temperature, which is attributable to the combination of the homopolymer and the backbone gelatin; and (3) generally, the number of branches was small and the molecular weight of the branched polymer was high in this polymerization.
Bacterial Polyesters
The natural polyesters, which are produced by a wide variety of bacteria as intracellular reserve materials, are receiving increased attention for possible applications as biodegradable, melt processable polymers which can be produced from renewable resources. The members of this family of thermoplastic biopolymers can show variation in their material properties from rigid brittle plastics, to flexible plastics with good impact properties to strong tough elastomers, depending on the size of the pendant alkyl group, R, and the composition of the polymer.
All of these polyesters contain units which are 100% optically pure at the β-position, so all are 100% isotactic. The polymer with R=CH3, poly-β-hydroxybutyrate (PHB), is highly crystalline with a melting temperature of 180°C and a glass transition temperature, Tg, of approximately 5°C. This combination of very high crystallinity and relatively high Tg makes the films and plastics of PHB very brittle, so copolymers with units containing other alkyl groups, especially R=C2H5, are preferred. All of these materials are inherently biodegradable. Polyesters with longer alkyl substituents are also produced by a variety of bacteria, generally in the form of copolymers which have lower degrees of crystallinity and lower Tm and Tg values. As a result, these longer alkyl chain polyesters are useful as thermoplastic elastomers, which can have excellent strength and toughness, and yet are also inherently biodegradable.
Considerable interest arose when a large-scale, controlled fermentation process was developed for the production of copolymers of PHB. Feeding the bacteria with a variety of carbon sources led to the production of different copolymers and a material was obtained with better mechanical properties than PHB. The biodegradation of PHB and its copolymers has been studied in environments such as soil, activated sludge and sea water.
The native polyesters are also hydrolyzed in water at a very slow rate. In vivo this is the main degradation mechanism, involving chain scission of the polymer. The hydrolytic degradation of hydroxybutyrate–hydroxyvalerate copolymers in vitro begins with a surface modification, accompanied by water diffusion into the matrix. A progressive increase in porosity facilitates the diffusion by removal of degradation products.
Characterization
There are a number of biophysical techniques for determining sequence information. Protein sequence can be determined by Edman degradation, in which the N-terminal residues are hydrolyzed from the chain one at a time, derivatized, and then identified. Mass spectrometer techniques can also be used. Nucleic acid sequence can be determined using gel electrophoresis and capillary electrophoresis. Lastly, mechanical properties of these biopolymers can often be measured using optical tweezers or atomic force microscopy. Dual polarisation interferometry can be used to measure the conformational changes or self assembly of these materials when stimulated by pH, temperature, ionic strength or other binding partners. [9] [10] [11]
Applications
The applications of biodegradable polymers have been focused on three major areas: medical, agricultural, and consumer goods packaging. Some of these have resulted in commercial products. Because of their specialized nature and greater unit value, medical device applications have developed faster than the other two.
This section provides an overview of special applications in the following areas: electronics, photonics, aerospace, medicine and pharmacy, food and agriculture, packaging, construction engineering, etc. Further topics included are: biotechnological production of monomers for chemical polymer synthesis, conversion of raw materials, corrosion, composing, environmental impacts, health issues, legal, ecological and economic aspects. [12] [13] [14] [15]
Plastics
Plastic filler
Starch has been used for many years as an additive to plastic for various purposes. Starch was added as a filler to various resin systems to make films that were impermeable to water but permeable to water vapour. Starch as a biodegradable filler in LDPE was reported. A starch-filled polyethylene film was prepared which becomes porous after the extraction of the starch. This porous film can be readily invaded by microorganisms and rapidly saturated with oxygen, thereby increasing polymer degradation by biological and oxidative pathways.
Since isocyanates are highly reactive with hydroxyl groups, they can be used to prepare a number of reactive resins that crosslink with starch. The addition of starch to isocyanate resins considerably reduced costs and improved solvent resistance and strength properties. Starch can be modified with nonpolar groups, such as fatty esters, before the isocyanate reaction to improve the degree of reactivity. A method was developed to incorporate starch as a filler and crosslinking agent in diisocyanate-modified polyesters to yield elastomers.
Some biopolymers- such as polylactic acid (PLA), naturally occurring zein, and poly-3-hydroxybutyrate can be used as plastics, replacing the need for polystyrene or polyethylene based plastics. Some plastics are now referred to as being 'degradable', 'oxy-degradable' or 'UV-degradable'. This means that they break down when exposed to light or air, but these plastics are still primarily (as much as 98 per cent) oil-based and are not currently certified as 'biodegradable' under the European Union directive on Packaging and Packaging Waste (94/62/EC). Biopolymers, however, will break down and some are suitable for domestic composting.
Packaging
Many types of packaging can be made from biopolymers: food trays, blown starch pellets for shipping fragile goods, thin films for wrapping. Physical characteristics of 'packaging polymers' are greatly influenced by the chemical structure, molecular weight, crystallinity and processing conditions of the polymers used. The physical characteristics required in packaging depend on what item will be packaged as well as the environment in which the package will be stored. Items which must be kept frozen for a period of time require special packaging. Food items require more stringent packaging requirements than nonperishable goods.
The challenge in the development of biodegradable packaging will be to combine polymers which are truly biodegradable into a laminate film or a film blend which has properties as good as those found in synthetic laminates. For food applications, for example, it may be possible to coat food items with pullulan which has a very low oxygen permeability and is edible and to utilize PHBV as an outer packaging which has good flexibility and is a moisture barrier. A film blend of pullulan and PHBV can also be produced since both polymers can be melt blended under conditions where sufficient moisture is maintained during processing. The addition of pullulan to PHBV may reduce oxygen permeability and increase biodegradability of the blend due to the increased surface area of PHBV exposed following the rapid removal of pullulan due to its water solubility.
Several polysaccharide-based biopolymers are being used as possible coating materials or packaging films. They include starch, pullulan and chitosan. The degradation of synthetic polymer films can be accelerated by incorporating starch as a filler. LDPE blends with up to 10% corn starch were produced using conventional techniques and were made into bags for groceries or rubbish.
Pullulan is comprised primarily of maltotrise units connected by α-1,6 linkages. It is produced by several fungi as an extracellular secondary metabolite. Pullulan has been commercialized as a food source in Japan due to its natural origin and has been approved for food coatings. It is a water-soluble polymer which produces clear, edible films which exhibit low oxygen permeability. Films can be produced by casting a 1–20% aqueous solution of pullulan on a metal plate roller. Pullulan, like starch, can also be moulded with heat and pressure if sufficient water is present as a plasticizer.
Polylactic acid (PLLA) is formed by a chemical condensation reaction of the lactic acid monomer and has a tensile strength at break of 45–70 MPa and an elongation of 85–105%. Argonne National Laboratory has patented a process to produce glucose from potato starch in 10 h instead of 100 h. The glucose is then fermented to lactic acid and purified. The same laboratory estimates that the price for lactic acid produced from potato wastes is low enough to produce PLLA and manufacture biodegradable packaging items at a reasonable price. PLLA-based packaging under consideration include grocery and rubbish bags, diaper backings, six-pack rings and fast food containers.
Plant Containers
A small niche for degradable plastics is the use of polycaprolactone for small agricultural planting containers. Although this is a small volume application for degradable plastics, it is presented here because it is one of the few applications in which the polymer used is biodegradable within a reasonable period of time. These polycaprolactone planting containers have been used for automated machine planting of tree seedlings. Within six months in the soil, the polycaprolactone was found to undergo significant biodegradation, resulting in 48% weight loss, with 95% weight loss occuring in a year.
Medical
Biodegradable plastics have been developed as surgical implants in vascular and orthopedic surgery as implantable matrices for the controlled long-term release of drugs inside the body, as absorbable surgical sutures, and for use in the eye. Recently the term biomaterial was defined as a nonviable material used in medical device applications that is intended to interact with a biological system. It is also important to define biocompatibility, which deals with how the tissue reacts to foreign materials. Biocompatibility is the ability of a material to perform with an appropriate host response in a specific application. Biomaterials in general are used for the following purposes:
(a) to replace tissues that are diseased or otherwise nonfunctional, as in-joint replacements, artificial heart valves and arteries, tooth reconstruction and intraocular lenses;
(b) to assist in the repair of tissue, including the obvious sutures but also bone fracture plates, ligament and tendon repair devices;
(c) to replace all or part of the function of the major organs, such as in haemodialysis (replacing the function of the kidney), oxygenation (lungs), left ventricular or whole heart assistance (heart), perfusion (liver), and insulin delivery (pancreas);
(d) to deliver drugs to the body, either to targeted sites (e.g. directly to a tumour) or sustained delivery rates (insulin, pilocarpin, contraceptives).
Drug delivery
A new dimension for the use of polymeric materials as drug delivery devices involves incorporation of biodegradability into the system. A number of degradable polymers are potentially useful for this purpose, including a variety of synthetic and natural substances. The use of intentionally degradable polymers in medicine has been brought into prominence with new innovations in drug delivery systems. The limitations of conventional methods of drug delivery, by tablet or injection for example, are well known. As a dose is applied, the plasma levels will be raised but these will be rapidly decreased as the drug is metabolized and will soon be below therapeutic levels. The next dose takes the plasma level high again and a cyclical pattern may be established, with most of the drug plasma levels possibly being outside the optimal range. In addition, the drug usually permeates throughout the body and it is not targeted to the location where it is specifically required.
Amongst the many possible solutions to these problems is the use of controlled drug delivery systems, from which the drug is released at a constant, predetermined rate, and possibly targeted to a particular site. One of the most prominent approaches is that in which the drug is contained within a polymer membrane or is otherwise encapsulated in a polymer matrix and where the drug diffuses out into the tissues following implantation. In some cases, erosion or dissolution of the polymer contributes to the release mechanism. Degradable polymers such as poly(lactic acid) and poly(ortho ester)s, are used for drug delivery systems.
Certain soluble polymers may be used as carriers for targeted drugs. One group has reported the use of a variety of polymers to which are attached, via side groups, certain drugs which can be released following cleavage of the bonds attaching them to the backbone. The targeting or selectivity here is achieved by using bonds which are cleaved only under certain conditions, for example by liver enzymes, which permit the release of the drug only at their specific site of action.
The design of a plasticized, biodegradable polymeric material, suitable for application as a drug-delivery system, was attempted. A poly( -lactic acid) oligomer was plasticized with 1,2-propylene glycol and glycerol. The latter plasticizer showed poor compatibility whereas 1,2-propylene glycol was compatible with the polymer up to high concentrations. The mixtures prepared displayed considerable depression of processing temperatures and enhanced deliveries of salicylic acid in the early stages of release. It seemed, therefore, feasible to produce systems which allow easy and safe processing and can be injected into a body cavity without the need for surgical retrieval after completion of the release. Furthermore, the differential rates of drug delivery might be of profound interest for cases where elevated drug doses are necessary in the beginning of treatment.
Surgical sutures
Tissue damage which results in a loss of structural integrity, for example a deep cut in soft tissue or a fracture of a bone, may or may not be capable of unassisted self healing. The insertion of some material or device to hold the tissue together may facilitate the healing process. The classic examples are the use of sutures to hold both deep and superficial wounds together. Once the healing is complete, the suture becomes redundant and can impose undesirable constraints on the healing tissues. It is preferable to remove the material from the site, either physically or by degradation.
Synthetic absorbable sutures were developed in 1960s, and because of their good biocompatibility in tissues, they are now widely used in tracheobronchial surgery, as well as general surgery. They are multifilament-type sutures, which have good handleability. Polyglycolide (PGA), poly- -lactide (PLA) and their copolymers, and polyglactin are the most popular and are now commercially available. However, for continuous suturing, braided sutures with nonsmooth surfaces are not useful. Monofilament sutures have smooth surfaces and are adequate for continuous suturing. For a monofilament suture, PGA or PLA are too stiff and inflexible. The more flexible polydioxanones and polyglyconates can be used as sutures because of their lower bending moduli. Furthermore, copolymers of -lactide and ε-caprolactone-poly(CL-LA) are bioabsorbable elastic materials and their clinical applications have been studied.
Bone fixation
Although metal fixation in fracture treatment for undisturbed bone healing is a successful procedure, cortical bone and steel have very different mechanical properties. The elasticity constant of bone is only 1/10 that of implanted steel while tensile strength is 10 times lower. Thus, the removal of metal implants can result in weakened bone with a danger of refracture. Biodegradable implants can meet the dynamic processes of bone healing, decreasing the weight-bearing of the material. After months, the entire material will disappear completely and no secondary surgery is required. PGA, PLA, polydioxanone and PHD have potential roles in this area. For clinical applications, polydioxanone was recommended for ligament augmentation, for securing a ligament suture, as a kind of internal splinting suture and as a kind of internal splinting to allow for early motion of the extremities after an operation.
Biodegradable polymers are useful for many other applications. A marrow spacer can help to save autologous bone material. A plug for closing the bone marrow is employed for endoprosthetic joint replacement. Fibres are used for filling large bone defects without mechanical loads.
Vascular grafts
Many studies have been undertaken to develop acceptable small diameter vascular prostheses. One group designed small diameter vascular prostheses with incorporated matrices that can be absorbed into a growing anastomotic neointima. It was pointed out that a gelatin–heparin complex when adequately cross-linked, could simultaneously function as a temporary antithrombogenic surface and as an excellent substructure for an anastomotic neointima.
Anti-adhesion
Tissue adhesion after surgery occasionally causes serious complications. Materials that prevent tissue adhesion should be flexible and tough enough to provide for a tight cover over the traumatized soft tissues, and should be biodegradable and reabsorabable after the injured tissue is completely regenerated. One group developed photocurable mucopolysaccharides for a newly designed tissue adhesion prevention material that meets numerous requirements such as nonadherent surface characteristics, biocompatibility, biodegradability in accordance with the wound healing rate and nontoxicity.
Mucopolysaccharides (hyaluronic acid and chondronitin sulphate) partially functionalized with photoreactive groups, such as cinnamate or thymine, were subjected to UV irradiation to produce water-insoluble gels via intermolecular photodimerization of the photoreactive groups. Photocured films with lower degrees of substitution, which had high water swellability and flexibility, prevented tissue adhesion and exhibited enhanced biodegradability. It has been suggested that these newly designed mucopolysaccharide gels may aid injured tissue repair in a bioactive manner.
Artificial skin
For healing burns, skin substitutes or wound dressings made of biodegradable polymeric materials have recently been developed. Until now, most of the commercially developed artificial skins have utilized biodegradable polymers such as collagen, chitin and poly- -leucine, which are enzymatically degradable polymers.
One group developed a new type of biomaterial for artificial skin, in the form of a sponge, by combining fibrillar collagen (F-collagen) with gelatin. The sponge was physically and metabolically stabilized by introducing crosslinks. Although several types of collagen-based artificial skins have been developed, some undesirable characteristics of native collagen were noticed, such as inducing rodlike shapes in fibroblasts and enhancing the expression of collagenase genes in fibroblasts. F-collagen with gelatin was found to overcome the above problems.
Others developed a biosynthetic wound dressing with a drug delivery capability. This medicated wound dressing is composed of a spongy mixture sheet of chitosan-derivatized collagen, which is laminated with a gentamycin sulphate impregnated polyurethane membrane. From in vitro evaluation, it was shown that this wound dressing is capable of suppressing bacterial growth and minimizing cellular damage. Evaluation of this wound dressing was conducted in 80 clinical cases including superficial second-degree burns, deep second-degree burns, donor sites and pressure sores, and achieved excellent results.
The development of hybrid artificial skins is also another important target in biomedical engineering. Here, synthetic polymers and cell cultures are combined to form a synthetic–biological composite. In this case, a biodegradable polymer may be required as the template for growing cells and tissue cultures in vivo.
Agriculture
Since the introduction of plastic films in the 1930s and 1940s for greenhouse coverings, fumigation and mulching, agricultural applications of polymers have grown at an enormous rate. All principal classes of polymers, i.e. plastics, coatings, elastomers, fibres and water-soluble polymers are presently utilized in applications which include the controlled release of pesticides and nutrients, soil conditioning, seed coatings, gel plantings and plant protection. However, degradable plastics are also of interest as agricultural mulches and agricultural planting containers. Ultimate biodegradability, as in composting, is also of some interest as it would permit degradable plastics to be combined with other biodegradable materials and converted into useful soil-improving materials.
Mulching films
Mulches permit growers to use plastic films to help with plant growth and then photodegrade in the fields thereby avoiding the cost of removal. The plastic films are desirable because they conserve moisture, reduce weeds and increase soil temperatures, thus improving the rate of growth in plants. For example, a 6 ha melon farm reported a two- to three-fold increase in yield and ripening two weeks earlier as a result of using black polyethylene mulch. Elimination of weeds and avoidance of soil compaction by the use of mulch eliminates the need for cultivation, therefore root damage and stunting or killing of plants is further avoided. Fertilizer and water requirements are also reduced.
Researchers found that a starch–polyvinyl alcohol film could be coated with a thin layer of water-resistant polymer to give a degradable agricultural mulching film. Starch-based polyethylene films were formulated and consisted of up to 40% starch, urea, ammonia and various portions of low-density polyethylene (LDPE) and poly(ethylene-co-acrylic acid) (EAA). The EAA acted as a compatibilizer, forming a complex between the starch and the PE in the presence of ammonia. The resulting blend could be cast or blown into films, and had physical properties approaching those of LDPE.
In one trial, three techniques were used to incorporate large amounts of starch as a filler into disposable polyvinyl chloride (PVC) plastics. In the first technique, a starch xanthate solution was prepared by mixing starch with aqueous NaOH and then adding a small amount of carbon disulphide (usually 0.1 mol CS2 per mol starch). To this starch–xanthate solution, a PVC latex was added. The starch–xanthate and PVC resins were then co-precipitated by adding NaNO2 and alum. The fine powder obtained from this was blended with dioctyl phthalate (DOP). In the second technique (a concentration method), whole starch was gelatinized by heating in water before mixing into the PVC latex. After removing the water, dry product was mixed with DOP. In the third method, starch was dry-blended with PVC and DOP. These films appear to be useful for a variety of agricultural applications.
In addition, transparent polyethylene is more effective in trapping heat than black or smoke-grey films. Soil temperatures may rise 5.5°C under clear films, as compared to 1.7 – 2.7°C under black films. Radiative heat loss at night, as the soil cools, is lessened by polymer films. In some cases, weed control has been reported because of solar heating of the polyethylene mulches.
If left in place, however, conventional films can cause problems during harvesting or during cultivating operations the next year. Removal and disposal are costly and inconvenient. Therefore, interest in the development of biodegradable or photodegradable films with short service lifetimes has grown. Although a large number of polymer types could be designed for controlled degradation, only a few have been commercialized. Plastics used for this purpose usually contain light-sensitizing additives which cause the materials to undergo photodegradation.
The plastics used for mulch films are generally low density polyethylenes, poly(vinyl chloride), polybutylene or copolymers of ethylene with vinyl acetate. A particularly interesting photodegradable system consists of a mixture of ferric and nickel dibutyldithiocarbamates, the ratio of which is adjusted to provide protection for specific growing periods. The degradation is tuned so that when the growing season is over the plastic will begin to photodegrade. Another additive system being proposed for this application includes a combination of substituted benzophenones and titanium or zirconium chelates. The principal commercial degradable mulch is photodegradable poly(1-butene).
Biodegradable films based on starch with poly(vinyl alcohol) poly(ethylene-co-acrylic acid) and poly(vinyl chloride) have been developed in the USDA laboratories. Polylactone and poly(vinyl alcohol) films are readily degraded by soil microorganisms, whereas the addition of iron or calcium accelerated the breakdown of polyethylene. Degradable mulches should break down into small brittle pieces which pass through harvesting machinery without difficulty and do not interfere with subsequent planting.
Effective fumigant mulches require reduced-porosity films which reduce the escape of volatile chemicals, i.e. nematocides, insecticides, herbicides, etc., and therefore allow for lower application rates.
Controlled release
Controlled release (CR) is a method by which biologically active chemicals are made available to a target species at a specified rate and for a predetermined time. The polymer serves primarily to control the rate of delivery, mobility, and period of effectiveness of the chemical component. The principal advantage of CR formulations is that less chemicals are used for a given time period, thus lowering the impact on non-target species and limiting leaching, volatilization, and degradation. The macromolecular nature of polymers is the key to limiting chemical losses by these processes.
CR polymeric systems can be divided into two broad categories. In the first, the active agent is dissolved, dispersed, or encapsulated by the polymeric matrix or coating. Release generally occurs by diffusion processes or by the biological or chemical breakdown of the matrix. In the second category polymers contain the active agent as part of the macromolecular backbone or pendent side chain. Release results from biological or chemical cleavage of the bond between the bioactive agents and the polymer.
Physical systems that incorporate agricultural chemicals include microcapsules, physical blends, dispersions in plastics, laminates, hollow fibers and membranes. Kinetic models for release have been developed for each type of device.
Starch, cellulose, chitin, alginic acid, and lignin are among the natural polymers used in CR systems. These have the advantages of being abundant, relatively inexpensive, and biodegradable. Although they possess functionality for derivatization, they have the one significant disadvantage of being insoluble in standard solvents suitable for encapsulation, dispersion, and formulation. Systems have been developed that overcome the solvent problem by in situ encapsulation, whereby gelantinized starch containing a chosen pesticide is cross-linked by adding calcium chloride or boric acid, or by xanthanation followed by oxidation. The pesticide, as a result, is entrapped within the granular particles formed.
One of the largest applications for CR technology in agriculture is with fertilizers. E.G. Urea, a significant nitrogen source, easily reacts with formaldehyde to form a polymer. Subsequent hydrolysis of the polymer yields urea. Therefore, this is a simple and inexpensive system for CR.
Biodegredation factors
Polymer structure
Natural macromolecules, e.g. protein, cellulose, and starch are generally degraded in biological systems by hydrolysis followed by oxidation. It is not surprising, then, that most of the reported synthetic biodegradable polymers contain hydrolyzable linkages along the polymer chain. E.G. Amide enamine, ester, urea, and urethane linkages are susceptible to biodegradation by microorganisms and hydrolytic enzymes. Since many proteolytic enzymes specifically catalyze the hydrolysis of peptide linkages adjacent to substituents in proteins, substituted polymers containing substituents such as benzyl, hydroxy, carboxy, methyl, and phenyl groups have been prepared in the hope that an introduction of these substituents might increase biodegradability. Among benzylated polymers, mixed results have been obtained for polyamides. Apparently, the chiral specificity of enzymes are maintained here.
Since most enzyme-catalyzed reactions occur in aqueous media, the hydrophilic–hydrophobic character of synthetic polymers greatly affects their biodegradabilities. A polymer containing both hydrophobic and hydrophilic segments seems to have a higher biodegradability than those polymers containing either hydrophobic or hydrophilic structures only.
In order for a synthetic polymer to be degradable by enzyme catalysis, the polymer chain must be flexible enough to fit into the active site of the enzyme. This most likely accounts for the fact that, whereas the flexible aliphatic polyesters are readily degraded by biological systems, the more rigid aromatic polymer compound is generally considered to be bioinert.
Polymer morphology
One of the principal differences between proteins and synthetic polymers is that proteins do not have equivalent repeating units along the polypeptide chains. This irregularity results in protein chains being less likely to crystallize. It is quite probable that this property contributes to the ready biodegradability of proteins. Synthetic polymers, on the other hand, generally have short repeating units. This regularity enhances crystallization, making the hydrolyzable groups inaccessible to enzymes. It was reasoned that synthetic polymers with long repeating units would be less likely to crystallize and thus might be biodegradable. Indeed, a series of polymers were found to be readily degraded by subtilisin.
Selective chemical degradation of semi-crystalline polymer samples shows certain characteristic changes. During degradation, the crystallinity of the sample increases rapidly at first, then levels off to a much slower rate as the crystallinity approaches 100%. This is attributed to the eventual disappearance of the amorphous portions of the sample. The effect of morphology on the microbial and enzymatic degradation of PCL, a known biodegradable polymer with a number of potential applications, has been studied.
Scanning electron microscopy (SEM) has shown that the degradation of a partially crystalline polycaprolactone film by filamentous fungi proceeds in a selective manner, with the amorphous regions being degraded prior to the degradation of the crystalline region. The microorganisms produce extracellular enzymes responsible for the selective degradation. This selectivity can be attributed to the less-ordered packing of amorphous regions, which permits easier access for the enzyme to the polymer chains. The size, shape and number of the crystallites all have a pronounced effect on the chain mobility of the amorphous regions and thus affect the rate of the degradation. This has been demonstrated by studying the effects of changing orientation via stretching on the degradation.
Biodegradation proceeds differently from chemical degradation. Studies on the degradation by solutions of 40% aqueous methylamine have shown a difference in morphology and molecular weight changes and in the ability of the degrading agents to diffuse into the substrate. Also, it was found that the differences in degradation rates between amorphous and crystalline regions are not same. The enzyme is able to degrade the crystalline regions faster than can methylamine. Quantitative GPC (gel permeation chromatography) analysis shows that methylamines degrade the crystalline regions, forming single and double transverse length products.
The enzyme system, on other hand, shows no intermediate molecular weight material and much smaller weight shift with degradation. This indicates that although degradation is selective, the crystalline portions are degraded shortly after the chain ends are made available to the exoenzyme. The lateral size of the crystallites has a strong effect on the rate of degradation because the edge of the crystal is where degradation of the crystalline material takes place, due to the crystal packing. A smaller lateral crystallite size yields a higher crystallite edge surface in the bulk polymer. Prior to the saturation of the enzyme active sites, the rate is dependent on available substrate. Thus a smaller lateral crystallite size results in a higher rate of degradation.
The degradation rate of a PCL film is zero order with respect to the total polymer, but is not zero order with respect to the concentrations of the crystallite edge material. The drawing of PCL films causes an increase in the rate of degradation, whereas annealing of the PCL causes a decrease in the rate of degradation. This is probably due to opposite changes in lateral crystallite sizes.
In vitro chemical and enzymatic degradations of polymers, especially polyesters, were analyzed with respect to chemical composition and physical properties. It was found quite often that the composition of a copolymer giving the lowest melting point is most susceptible to degradation. The lowest packing order, as expected, corresponds with the fastest degradation rate.
Effects of radiation
Photolysis with UV light and the γ-ray irradiation of polymers generate radicals and/or ions that often lead to cleavage and crosslinking. Oxidation also occurs, complicating the situation, since exposure to light is seldom in the absence of oxygen. Generally this changes the material's susceptibility to biodegradation. Initially, one expects the observed rate of degradation to increase until most of the fragmented polymer is consumed and a slower rate of degradation should follow for the crosslinked portion of the polymer. A study of the effects of UV irradiation on hydrolyzable polymers confirmed this. Similarly, photooxidation of polyalkenes promotes (slightly in most cases) the biodegradation. The formation of carbonyl and ester groups is responsible for this change.
Processes have been developed to prepare copolymers of alkenes containing carbonyl groups so they will be more susceptible to photolytic cleavage prior to degradation. The problem with this approach is that negligible degradation was observed over a two year period for the buried specimens. Unless a prephotolysis arrangement can be made, the problem of plastic waste disposal remains serious, as it is undesirable to have open disposal, even with constant sunlight exposure.
As expected, γ-ray irradiation greatly affects the rate of in vitro degradation of polyesters, where the pH of the degradation solution decreased as the process proceeded. The change-time curves exhibits sigmoidal shapes and consist of three stages: early, accelerated, and later. The lengths of these three regions vary with γ-ray irradiation. Increasing radiation dosage shortens the time of the early stage. The appearance of the drastic pH changes coincides with loss of tensile breaking strength. Similar effects via enzymatic and microbial degradation remain to be demonstrated.
Molecular weight
There have been many studies on the effects of molecular weight on biodegradation processes. Most of the observed differences can be attributed to the limit of detecting the changes during degradation, or, even more often, the differences in morphology and hydrophilicity–hydrophobicity of polymer samples of varying molecular weight. Microorganisms produce both exoenzymes (degrading polymers from terminal groups inwards) and endoenzymes (degrading polymers randomly along the chain). One might expect a large molecular effect on the rate of degradation in the ease of exoenzymes and a relatively small molecular weight effect in the case of endoenzymes.
Plastics remain relatively immune to microbial attack as long as their molecular weight remains high. Many plasticsdo not support microbial growth. Low molecular weight hydrocarbons, however, can be degraded by microbes. They are taken in by microbial cells, ‘activated' by attachment to coenzyme-A, and converted to cellular metabolites within the microbial cell. However, these processes do not function well (if at all) in an extracellular environment, and the plastic molecules are too large to enter the cell. This problem does not arise with natural molecules, such as starch and cellulose, because conversions to low molecular weight components by enzyme reactions occur outside the microbial cell. However, photodegradation or chemical degradation may decrease molecular weight to the point that microbial attack can proceed.
Biodegradation modes
The biological environment, i.e. the biological surroundings in which polymers are present, includes the biological agents responsible for the deterioration of polymeric substances. Biological agents such as bacteria, fungi and their enzymes consume a substance as a food source so that its original form disappears. Under appropriate conditions of moisture, temperature, and oxygen availability, biodegradation is a relatively rapid process.
Microorganisms
Two types of microorganisms are of particular interest in the biodegradation of natural and synthetic polymers: bacteria and fungi.
Fungi
Eumycetes, or true fungi, are microorganisms of particular importance in causing the degradation of materials. Fungi are nucleated, spore-forming, nonchlorophyllous organisms, which reproduce both sexually and asexually; most of them possess filamentous, somatic structures, and cell walls of chitin and/or cellulose. More than 80 000 species are known.
True fungi are present everywhere. Their importance as deteriorative agents is a result of the production of enzymes which break down nonliving substrates in order to supply nutrient materials present in polymer compositions. Certain environmental conditions are essential for optimum growth and degradative activity. These include an optimal ambient temperature, the presence of nutrient materials, and high humidity.
The group of test fungi that evolved for assay purposes in the field of natural polymers and that were further selected for their utility in assay procedures on synthetic polymers are taxonomically a very heterogeneous group, exhibiting no marked taxonomic similarities among them (for example based on morphology). Many of them were selected primarily because their reproduction spores are produced asexually and the variation associated with spores resulting from the fusion of sexual element is minimized. The most acceptable organisms are characterized by strain or culture collection number.
Bacteria
Schizomycetes, a bacteria, have played an undetermined role in relation to fungi in polymer deterioration. Bacteria can be single-cell rods, cocci, or spirilla. Others are chain-like or filamentous. Bacteria can either be aerobic or anaerobic. In contrast, fungi are necessarily aerobic. Some bacteria are motile, while others are predominantly nonchlorophyllous. Their degradative action is also chiefly a result of enzyme production and resultant breakdown of the nonliving substrate in order to obtain nutrient materials.
Bacteria present in soil are important agents for material degradation. Particularly affected are cellulosic plant life, wood products, and textiles subject to cellulytic degradation.
Enzymes
Enzymes are essentially biological catalysts, with the same action as chemical catalysts. By lowering the activation energy they can induce an increase in reaction rates in an environment otherwise unfavorable for chemical reactions (e.g. water at pH 7 and 30°C). In the presence of enzymes, a rise in reaction rate of 108–1020 can often be observed. The vast majority of enzymes are proteins having a polypeptide chain with a complex three-dimensional structure. Enzyme activity is closely related to conformational structure.
The three-dimensional structure of enzymes with folds and pockets creates certain regions on the surface with characteristic primary structures (i.e. specific amino acid sequences) which form an active site. At the active site the interaction between the enzyme and substrate takes place leading to a chemical reaction, giving a particular product.
For optimal activity certain enzymes must associate with cofactors which can be metal ions, e.g. sodium, potassium, magnesium, calcium or zinc. Organic cofactors are also called coenzymes and they can vary in structure. Some are derived from different B-vitamins (thiamine, biotin, etc.) while others are important compounds in metabolic cycles.
For enzymes with absolute specificities the ‘key-and-lock' theory which implies an unchangeable rigid conformation, is a plausible model. The initial contact between an enzyme and substrate forms an optimal orientation at the active site giving good possibilities for maximum bonding (enzyme–substrate), often the cofactor induces these changes when binding to the enzyme.
Enzyme activity
All enzymes are adjusted to a specific environment in which their activity and three-dimensional structure are optimal for a specific purpose. For human enzymes or enzymes isolated from human cells, this environment is a water solution at pH 6–8, an ion strength of 0.15 molar (as is normal physiological saline at 0.9% NaCl) and a temperature of 35–40°C. An extremely small change one of these parameters may render the enzyme totally inactive and sometimes can even destroy it irreversibly. Other solvents than water, especially organic solvents, are also lethal to many enzymes but, on other hand, there are enzymes that are active in extreme environments, e.g. in hot water springs or salty environments.
Different enzymes have different actions, some enzyme change the substrate through a free radical mechanism while others follow alternative chemical routes. Typical examples are biological oxidation and biological hydrolysis.
Several enzymes can react directly with oxygen, the classical example being cytochromoxidase which is active in the respiratory chain. Oxygen has a special role in the metabolism of aerobic organisms. In many cases oxygen is directly incorporated into the substrate. The enzyme can be either hydroxylases or oxygenases.
See also
- Biomaterials
- Bioplastics
- Biopolymers & Cell (journal)
- Polymer chemistry
- Condensation polymers
- DNA sequence
- Melanin
- Non food crops
- Phosphoramidite
- Small molecules
- Sequencing
- Worm-like chain
References
- ^ Mohanty, A.K., et al., Natural Fibers, Biopolymers, and Biocomposites (CRC Press, 2005)
- ^ Chandra, R., and Rustgi, R., "Biodegradable Polymers", Progress in Polymer Science, Vol. 23, p. 1273 (1998)
- ^ Meyers, M.A., et al., "Biological Materials: Structure & Mechanical Properties", Progress in Materials Science, Vol. 53, p. 1 (2008)
- ^ Kumar, A., et al., "Smart Polymers: Physical Forms & Bioengineering Applications", Progress in Polymer Science, Vol. 32, p.1205 (2007)
- ^ Klemm, D., Heublein, B., Fink, H., and Bohn, A., "Cellulose: Fascinating Biopolymer / Sustainable Raw Material", Ang. Chemie (Intl. Edn.) Vol. 44, p. 3358 (2004)
- ^ Stevens, E.S., Green Plastics: An Introduction to the New Science of Biodegradable Plastics, Princeton Univ. Press (2001)
- ^ Y. Roiter and S. Minko (2005). "AFM Single Molecule Experiments at the Solid-Liquid Interface: In Situ Conformation of Adsorbed Flexible Polyelectrolyte Chains". Journal of the American Chemical Society. 127 (45): 15688–15689. doi:10.1021/ja0558239. PMID 16277495.
- ^ Stupp, S.I and Braun, P.V., "Role of Proteins in Microstructural Control: Biomaterials, Ceramics & Semiconductors", Science, Vol. 277, p. 1242 (1997)
- ^ Van Der Maarel, J.R.C., Introduction To Biopolymer Physics (World Scientific Publishing Co., 2007)
- ^ Sperling, L.H., Introduction to Physical Polymer Science, (John Wiley & Sons, Inc., 1986)
- ^ Saito, H., et al., Solid State NMR Spectroscopy for Biopolymers: Principles and Applications (Springer, 2006)
- ^ Kasapis, S., Norton, I.T. and Ubbink, J.B., Eds., Modern Biopolymer Science: Bridging the Divide between Fundamental Treatise and Industrial Application (Academic Press, 2009)
- ^ Vekshin, N.L., Photonics of Biopolymers (Springer, 2002)
- ^ Steinbuchel, A., Biopolymers: General Aspects and Special Applications, (Wiley - VCH 2003)
- ^ Dickinson, E. and van Vliet, T., Food Colloids, Biopolymers and Materials Royal Society of Chemistry, Great Britain, 2003)
