Voltammetry: Difference between revisions
m Typo and General fixing, replaced: ground work → groundwork using AWB |
No edit summary |
||
| Line 100: | Line 100: | ||
*[[Reference electrode]] |
*[[Reference electrode]] |
||
*[[Auxiliary electrode]] |
*[[Auxiliary electrode]] |
||
*[[Neopolarogram]] |
|||
==References== |
==References== |
||
Revision as of 16:38, 6 March 2011


Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied.[1][2]
Three electrode system
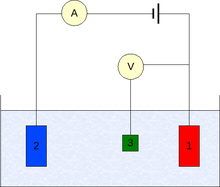
Voltammetry experiments investigate the half cell reactivity of an analyte. Most experiments control the potential (volts) of an electrode in contact with the analyte while measuring the resulting current (amperes).[3]
To conduct such an experiment requires at least two electrodes. The working electrode, which makes contact with the analyte, must apply the desired potential in a controlled way and facilitate the transfer of charge to and from the analyte. A second electrode acts as the other half of the cell. This second electrode must have a known potential with which to gauge the potential of the working electrode, furthermore it must balance the charge added or removed by the working electrode. While this is a viable setup, it has a number of shortcomings. Most significantly, it is extremely difficult for an electrode to maintain a constant potential while passing current to counter redox events at the working electrode.
To solve this problem, the role of supplying electrons and referencing potential has been divided between two separate electrodes. The reference electrode is a half cell with a known reduction potential. Its only role is to act as reference in measuring and controlling the working electrodes potential and at no point does it pass any current. The auxiliary electrode passes all the current needed to balance the current observed at the working electrode. To achieve this current, the auxiliary will often swing to extreme potentials at the edges of the solvent window, where it oxidizes or reduces the solvent or supporting electrolyte. These electrodes, the working, reference, and auxiliary make up the modern three electrode system.
There are many systems which have more electrodes, but their design principles are generally the same as the three electrode system. For example, the rotating ring-disk electrode has two distinct and separate working electrodes, a disk and a ring, which can be used to scan or hold potentials independently of each other. Both of these electrodes are balanced by a single reference and auxiliary combination for an over all four electrode design. More complicated experiments may add working electrodes as required and at times reference or auxiliary electrodes.
In practice it can be very important to have a working electrode with known dimensions and surface characteristics. As a result, it is common to clean and polish working electrodes regularly. The auxiliary electrode can be almost anything as long as it doesn't react with the bulk of the analyte solution and conducts well. The reference is the most complex of the three electrodes, there are a variety of standards used and its worth investigating elsewhere. For non-aqueous work, IUPAC recommends the use of the ferrocene/ferrocenium couple as an internal standard[citation needed]. In most voltammetry experiments a bulk electrolyte (also known as supporting electrolyte) is used to minimize solution resistance. It can be possible to run an experiment without a bulk electrolyte but the added resistance greatly reduces accuracy of the results. In the case of room temperature ionic liquids the solvent can act as the electrolyte.
Theory
Data analysis requires consideration of kinetics in addition to thermodynamics due to the temporal component of voltammetry. Idealized theoretical electrochemical thermodynamic relationships such as the Nernst equation are modeled without a time component. While these models are insufficient alone to describe the dynamic aspects of voltammetry, models like the Nernst equation and Butler-Volmer equation lay the groundwork for the modified voltammetry relationships that relate theory to observed results.[4]
Types of voltammetry
- Linear sweep voltammetry
- Staircase voltammetry
- Squarewave voltammetry
- Cyclic voltammetry - A voltammetric method that can be used to determine diffusion coefficients and half cell reduction potentials.
- Anodic stripping voltammetry - A quantitative, analytical method for trace analysis of metal cations. The analyte is deposited (electroplated) onto the working electrode during a deposition step, and then oxidized during the stripping step. The current is measured during the stripping step.
- Cathodic stripping voltammetry - A quantitative, analytical method for trace analysis of anions. A positive potential is applied, oxidizing the mercury electrode and forming insoluble precipitates of the anions. A negative potential then reduces (strips) the deposited film into solution.
- Adsorptive stripping voltammetry - A quantitative, analytical method for trace analysis. The analyte is deposited simply by adsorption on the electrode surface (i.e., no electrolysis), then electrolyzed to give the analytical signal. Chemically modified electrodes are often used.
- Alternating current voltammetry
- Polarography - a subclass of voltammetry where the working electrode is a dropping mercury electrode (DME), useful for its wide cathodic range and renewable surface.
- Rotated electrode voltammetry - A hydrodynamic technique in which the working electrode, usually a rotating disk electrode (RDE) or rotating ring-disk electrode (RRDE), is rotated at a very high rate. This technique is useful for studying the kinetics and electrochemical reaction mechanism for a half reaction.
- Normal pulse voltammetry
- Differential pulse voltammetry
- Chronoamperometry
History
The beginning of voltammetry was facilitated by the discovery of polarography in 1922 by the Nobel Prize winning chemist Jaroslav Heyrovský. Early voltammetric techniques had many problems, limiting their viability for everyday use in analytical chemistry. In 1942 Hickling built the first three electrodes potentiostat.[5] The 1960s and 1970s saw many advances in the theory, instrumentation, and the introduction of computer added and controlled systems. These advancements improved sensitivity and created new analytical methods. Industry responded with the production of cheaper potentiostat, electrodes, and cells that could be effectively used in routine analytical work.
Applications
Voltametric sensors A number of voltammetric systems are produced commercially for the determination of specific species that are of interest in industry and research. These devices are sometimes called electrodes but are, in fact, complete voltammetric cells and are better referred to as sensors.
The oxygen electrode The determination of dissolved oxygen in a variety of aqueous environments, such as sea water, blood, sewage, effluents from chemical plants, and soils is of tremendous importance to industry, biomedical and environmental research, and clinical medicine. One of the most common and convenient methods for making such measurements is with the Clark oxygen sensor, which was patented by L.C. Clark, Jr. in 1956.
See also
- Electroanalytical method
- Cyclic voltammetry
- Working electrode
- Reference electrode
- Auxiliary electrode
- Neopolarogram
References
- ^ Kissinger, Peter (1996-01-23). Laboratory Techniques in Electroanalytical Chemistry, Second Edition, Revised and Expanded (2 ed.). CRC. ISBN 0824794451.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zoski, Cynthia G. (2007-02-07). Handbook of Electrochemistry. Elsevier Science. ISBN 0444519580.
- ^ Bard, Allen J. (2000-12-18). Electrochemical Methods: Fundamentals and Applications (2 ed.). Wiley. ISBN 0471043729.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Nicholson, R. S. (1964-04-01). "Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems". Analytical Chemistry. 36 (4): 706–723. doi:10.1021/ac60210a007.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|unused_data=ignored (help) - ^ Hickling, A. (1942). "Studies in electrode polarisation. Part IV.-The automatic control of the potential of a working electrode". Transactions of the Faraday Society. 38: 27–33. doi:10.1039/TF9423800027.
{{cite journal}}:|access-date=requires|url=(help)
Further reading
- Reinmuth, W. H. (1961-11-01). "Theory of Stationary Electrode Polarography". Analytical Chemistry. 33 (12): 1793–1794. doi:10.1021/ac60180a004.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|unused_data=ignored (help) - Skoog, Douglas A. (1995-08-25). Fundamentals of Analytical Chemistry (7th ed.). Harcourt Brace College Publishers. ISBN 0030059380.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Zanello, P. (2003-10-01). Inorganic Electrochemistry: Theory, Practice, and Application (1 ed.). Royal Society of Chemistry. ISBN 0854046615.
External links
- http://www.drhuang.com/science/chemistry/electrochemistry/polar.doc.htm
- http://www.autolab-instruments.com/download/content/Appl021.pdf
- http://www.amelchem.com/download/items/voltammetry/manuals/eng/manual_eng.pdf
- http://new.ametek.com/content-manager/files/PAR/App%20Note%20E-4%20-%20Electrochemical%20Analysis%20Techniques1.pdf
- http://www.prenhall.com/settle/chapters/ch37.pdf
