Clemmensen reduction: Difference between revisions
explanation |
|||
| Line 3: | Line 3: | ||
[[Image:Clemmensen Reduction Scheme.png|center|300px|The Clemmensen reduction]] |
[[Image:Clemmensen Reduction Scheme.png|center|300px|The Clemmensen reduction]] |
||
The Clemmensen reduction is particularly effective at reducing [[aryl]]-[[alkyl]] ketones |
The Clemmensen reduction is particularly effective at reducing [[aryl]]-[[alkyl]] ketones<ref>{{OrgSynth|prep=cv2p0499|title=γ-Phenylbutyric acid|volume=2|page=499|year=1943}}; Vol. 15, p.64 (1935)</ref><ref>{{OrgSynth|prep=cv4p0203|title=Creosol|volume=4|page=203|year=1963}}; Vol. 33, p.17 (1953).</ref>, such as those formed in a Friedel-Crafts acylation. With [[aliphatic]] or cyclic ketones, [[zinc]] metal reduction is much more effective.<ref>{{OrgSynth|prep=cv6p0289|title= Modified Clemmensen Reduction: Cholestane|volume=6|page=289|year=1988}}; Vol. 53, p.86 (1973).</ref> |
||
The substrate must be stable in the strongly acidic conditions of the Clemmensen reduction. Acid sensitive substrates should be reacted in the [[Wolff-Kishner reduction]], which utilizes strongly basic conditions; a further, milder method is the [[Mozingo reduction]]. The oxygen atom is lost in the form of one molecule of water. |
The substrate must be stable in the strongly acidic conditions of the Clemmensen reduction. Acid sensitive substrates should be reacted in the [[Wolff-Kishner reduction]], which utilizes strongly basic conditions; a further, milder method is the [[Mozingo reduction]]. The oxygen atom is lost in the form of one molecule of water. |
||
Revision as of 15:41, 11 August 2013
Clemmensen reduction is a chemical reaction described as a reduction of ketones (or aldehydes) to alkanes using zinc amalgam and hydrochloric acid.[1][2][3] This reaction is named after Erik Christian Clemmensen, a Danish chemist.[4]
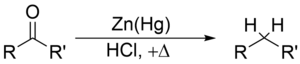
The Clemmensen reduction is particularly effective at reducing aryl-alkyl ketones[5][6], such as those formed in a Friedel-Crafts acylation. With aliphatic or cyclic ketones, zinc metal reduction is much more effective.[7]
The substrate must be stable in the strongly acidic conditions of the Clemmensen reduction. Acid sensitive substrates should be reacted in the Wolff-Kishner reduction, which utilizes strongly basic conditions; a further, milder method is the Mozingo reduction. The oxygen atom is lost in the form of one molecule of water.
References
- ^ Clemmensen, E. (1913). Chemische Berichte. 46: 1837.
{{cite journal}}: Missing or empty|title=(help) - ^ Clemmensen, E. (1914). Chemische Berichte. 47: 51.
{{cite journal}}: Missing or empty|title=(help) - ^ Clemmensen, E. (1914). Chemische Berichte. 47: 681.
{{cite journal}}: Missing or empty|title=(help) - ^ Biographies of Chemists, accessed 6 Feb 2007
- ^ "γ-Phenylbutyric acid". Organic Syntheses. 2: 499. 1943.; Vol. 15, p.64 (1935)
- ^ "Creosol". Organic Syntheses. 4: 203. 1963.; Vol. 33, p.17 (1953).
- ^ "Modified Clemmensen Reduction: Cholestane". Organic Syntheses. 6: 289. 1988.; Vol. 53, p.86 (1973).
Reviews
- Martin, E. L. (1942). Org. React. 1: 155.
{{cite journal}}: Missing or empty|title=(help) - Buchanan, J. G. St. C.; Woodgate, P. D. (1969). "The Clemmensen reduction of difunctional ketones". Quart. Rev. 23: 522. doi:10.1039/QR9692300522.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Vedejs, E. (1975). Org. React. 22: 40.
{{cite journal}}: Missing or empty|title=(help) - Yamamura, S.; Nishiyama, S. (1991). Comp. Org. Syn. 8: 309–313.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link)
