Essure: Difference between revisions
KolbertBot (talk | contribs) m Bot: HTTP→HTTPS (v485) |
Deleted a randomly placed "the" Tags: Mobile edit Mobile web edit |
||
| Line 24: | Line 24: | ||
}} |
}} |
||
'''Essure''' is a device for [[female sterilization]]. It is a metal coil which when placed into each fallopian tube induces fibrosis and blockage.<ref name="NEJM">{{cite journal| title =Revisiting Essure--Toward Safe and Effective Sterilization.| journal =N Engl J Med | date =Oct 8, 2015 | authors =Dhruva SS, Ross JS, Gariepy AM.| volume =373| issue =15| pages =e17| url =http://www.nejm.org/doi/full/10.1056/NEJMp1510514| doi =10.1056/NEJMp1510514| pmid =26397951| pmc =}}Free full text</ref> It was developed by [[Conceptus Inc.]] and [[US FDA]] approved in November 2002.<ref name="FDA"/> Conceptus was acquired by [[Bayer|Bayer AG]] of Germany in June 2013.{{cn|date=May 2018}}<ref>{{cite web|url=http://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/ucm371014.htm|title=Essure Permanent Birth Control|publisher=U.S. Food and Drug Administration|accessdate=June 24, 2014}}</ref> |
'''Essure''' is a device for [[female sterilization]]. It is a metal coil which when placed into each fallopian tube induces fibrosis and blockage.<ref name="NEJM">{{cite journal| title =Revisiting Essure--Toward Safe and Effective Sterilization.| journal =N Engl J Med | date =Oct 8, 2015 | authors =Dhruva SS, Ross JS, Gariepy AM.| volume =373| issue =15| pages =e17| url =http://www.nejm.org/doi/full/10.1056/NEJMp1510514| doi =10.1056/NEJMp1510514| pmid =26397951| pmc =}}Free full text</ref> It was developed by [[Conceptus Inc.]] and [[US FDA]] approved in November 2002.<ref name="FDA"/> Conceptus was acquired by [[Bayer|Bayer AG]] of Germany in June 2013.{{cn|date=May 2018}}<ref>{{cite web|url=http://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/ucm371014.htm|title=Essure Permanent Birth Control|publisher=U.S. Food and Drug Administration|accessdate=June 24, 2014}}</ref> |
||
Essure was designed as an alternative to [[tubal ligation]], the standard procedure and major surgery done in a hospital, because it can be placed in a doctor's office with less anesthesia. Although Essure was designed to remain in place for a lifetime, it was approved based on short-term safety studies. Of the 745 women with implants in the original premarket studies, only 92% were followed up at 1 year, and 25% for 2 years, for safety outcomes. |
Essure was designed as an alternative to [[tubal ligation]], the standard procedure and major surgery done in a hospital, because it can be placed in a doctor's office with less anesthesia. Although Essure was designed to remain in place for a lifetime, it was approved based on short-term safety studies. Of the 745 women with implants in the original premarket studies, only 92% were followed up at 1 year, and 25% for 2 years, for safety outcomes. |
||
| Line 33: | Line 33: | ||
In August 2017, the [[CE marking]] in the [[European Union]], and thus the commercial license for Essure was suspended for at least three months. Authorities in France and Ukraine recalled the implants, and the manufacturer withdrew the product voluntarily from the market in Canada, United Kingdom, Finland and the Netherlands.<ref>{{cite web|url=http://www.legalreader.com/essure-loses-commercial-license-in-european-union/|title=Essure Loses Commercial License in European Union - Legal Reader|first=Jay W. Belle|last=Isle|date=17 August 2017|website=Legalreader.com|accessdate=16 May 2018}}</ref><ref>{{cite web|url=http://birthcontrolproblems.com/eu-member-states-recall-essure/|title=Essure Sales Halted In European Union, Implants Recalled|date=7 August 2017|website=Birthcontrolproblems.com|accessdate=16 May 2018}}</ref><ref>{{cite web|url=https://medicalxpress.com/news/2017-08-eu-sale-contraceptive-implant.html|title=EU suspends sale of contraceptive implant|website=Medicalspress.com|accessdate=16 May 2018}}</ref><ref>{{cite web|url=https://yle.fi/uutiset/3-9780262|title=Satojen naisten sterilisaatiot menneet pieleen Suomessa – vaeltamaan lähteneitä implantteja löydetty muualta elimistöstä|website=Yle Uutiset|accessdate=16 May 2018}}</ref> |
In August 2017, the [[CE marking]] in the [[European Union]], and thus the commercial license for Essure was suspended for at least three months. Authorities in France and Ukraine recalled the implants, and the manufacturer withdrew the product voluntarily from the market in Canada, United Kingdom, Finland and the Netherlands.<ref>{{cite web|url=http://www.legalreader.com/essure-loses-commercial-license-in-european-union/|title=Essure Loses Commercial License in European Union - Legal Reader|first=Jay W. Belle|last=Isle|date=17 August 2017|website=Legalreader.com|accessdate=16 May 2018}}</ref><ref>{{cite web|url=http://birthcontrolproblems.com/eu-member-states-recall-essure/|title=Essure Sales Halted In European Union, Implants Recalled|date=7 August 2017|website=Birthcontrolproblems.com|accessdate=16 May 2018}}</ref><ref>{{cite web|url=https://medicalxpress.com/news/2017-08-eu-sale-contraceptive-implant.html|title=EU suspends sale of contraceptive implant|website=Medicalspress.com|accessdate=16 May 2018}}</ref><ref>{{cite web|url=https://yle.fi/uutiset/3-9780262|title=Satojen naisten sterilisaatiot menneet pieleen Suomessa – vaeltamaan lähteneitä implantteja löydetty muualta elimistöstä|website=Yle Uutiset|accessdate=16 May 2018}}</ref> |
||
In April 2018, the FDA restricted sale and use of Essure.<ref name=fda2018>US FDA [https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/EssurePermanentBirthControl/ucm452254.htm Medical Devices--FDA Activities: Essure] Page Last updated 04/09/2018, accessed 16 May 2018</ref> |
In April 2018, the FDA restricted sale and use of Essure.<ref name=fda2018>US FDA [https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/EssurePermanentBirthControl/ucm452254.htm Medical Devices--FDA Activities: Essure] Page Last updated 04/09/2018, accessed 16 May 2018</ref> |
||
==Device== |
==Device== |
||
Revision as of 07:17, 21 July 2018
| Essure | |
|---|---|
| Background | |
| Type | Sterilization |
| First use | 2002 |
| Failure rates (first year, after occlusion) | |
| Perfect use | 0.26% |
| Typical use | 0.26% |
| Usage | |
| Duration effect | Permanent |
| Reversibility | Irreversible |
| User reminders | Additional methods until 3 month check by hysterosalpingogram |
| Clinic review | None |
| Advantages and disadvantages | |
| STI protection | No |
| Benefits | Permanent contraception |
Essure is a device for female sterilization. It is a metal coil which when placed into each fallopian tube induces fibrosis and blockage.[1] It was developed by Conceptus Inc. and US FDA approved in November 2002.[2] Conceptus was acquired by Bayer AG of Germany in June 2013.[citation needed][3]
Essure was designed as an alternative to tubal ligation, the standard procedure and major surgery done in a hospital, because it can be placed in a doctor's office with less anesthesia. Although Essure was designed to remain in place for a lifetime, it was approved based on short-term safety studies. Of the 745 women with implants in the original premarket studies, only 92% were followed up at 1 year, and 25% for 2 years, for safety outcomes. A 2009 review concluded that Essure appeared safe and effective based on short-term studies, that it was less invasive and could be cheaper than laparoscopic bilateral tubal ligation.[4]
Since 2013, the product has been controversial, with thousands of women complaining of severe side effects leading to surgical extraction,[5] and campaigner Erin Brockovich has been hosting a website where women can share their stories after having the procedure.[5][6][7] As of 2015 a large number of adverse events, including tubal perforations, intractable pain and bleeding leading to hysterectomies, possible device-related deaths, and hundreds of unintended pregnancies occurred, according to the U.S. F.D.A. adverse events database and other studies.[1][8]
In August 2017, the CE marking in the European Union, and thus the commercial license for Essure was suspended for at least three months. Authorities in France and Ukraine recalled the implants, and the manufacturer withdrew the product voluntarily from the market in Canada, United Kingdom, Finland and the Netherlands.[9][10][11][12] In April 2018, the FDA restricted sale and use of Essure.[13]
Device
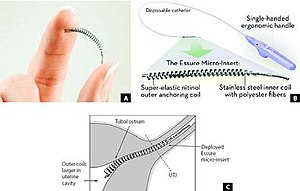
The small, flexible inserts are made from polyester fibers, nickel-titanium, stainless steel and solder. The insert contains inner polyethylene terephthalate fibers to induce inflammation, causing a benign fibrotic ingrowth,[14] and is held in place by flexible stainless steel inner coil and a dynamic outer nickel titanium alloy coil.[14] Unlike temporary methods of birth control, the Essure inserts do not contain or release hormones. The inserts do not prevent the transmission of sexually transmitted diseases.[citation needed]
Use
A physician places the coils into the fallopian tubes by a catheter passed from the vagina through the cervix and womb.[14] Once in place, the ingrowth continues over a period of three months, resulting in durable occlusion or blockage in the Fallopian tubes; the tissue barrier formed is supposed to prevent sperm from reaching an egg. During that intervening three month period, women are advised to use an alternate contraceptive method.[14]
Unlike other forms of tubal ligation, no general anaesthetic nor incision through the abdomen is required.[citation needed] Similar to some other methods of birth control, initially additional forms of birth control must be continued for 3 months[4] to prevent pregnancy until the method's effectiveness can be confirmed.
In one 2007 prospective study, the mean time for procedure was 6.8 minutes (range = 5–18 minutes).[14] for a trained physician to perform and can be performed in a physician's office.[citation needed] General anesthesia is not required. Despite this, some women have reported considerable pain during the procedure.[15]
Follow-up
For the Essure method, three months after insertion a radiologist is supposed to perform a fluoroscopic procedure called a hysterosalpingogram,[16] to confirm that the fallopian tubes are completely blocked and that the woman can rely on the Essure inserts for birth control. A contrast agent (dye) is injected through the cervix, and an x-ray technologist takes photos of the Essure coils to ensure no contrast leaks past the Essure.[2]
Efficacy
After successful insertion and occlusion, the Essure procedure is 99.74% effective based on 5 years of follow-up (although one study found failure rates closer to 11%).[17][18]
The reported insertional failure rates are "failure to place 2 inserts in the first procedure (5%), initial tubal patency (3.5%), expulsion (2.2%), perforation (1.8%), or other unsatisfactory device location (0.6%)".[19] Upon follow-up, occlusion was observed to have occurred in 96.5% of patients at 3 months with the remainder occluded by 6 months.[2] A 2015 study published in the BMJ concluded that Essure was as effuicacious as laparoscopic sterilization at preventing pregnancy, but with a "10-fold higher risk of undergoing re-operation" when compared to patients who underwent a laparoscopic sterilization procedure.[8]
Cautions and warnings
The procedure is reported to be permanent and not reversible by the manufacturing company. Nevertheless, several Essure reversals have been performed.[20]
Additional birth control must be used for three months after procedure until a hysterosalpingogram has been performed to confirm blockage.[19][21]
Because of the stainless steel medical staff needs to be notified before an MRI or any magnetic imaging can be done. [citation needed]
Risks
- Perforation, expulsion, or other unsatisfactory location of the insert
- Punctured uterine walls
- Pregnancy and increased risk of ectopic pregnancy
- Pain, cramping, vaginal bleeding, menstrual pattern changes, light periods at first then longer, heavier periods lasting up to 6–8 weeks due to changing birth control methods to a non-hormonal solution
- Nausea/vomiting
- Vasovagal response (fainting)
- Allergic reaction to the materials
- Heightened allergic response to other allergens
- heavy metal toxicity
- itchy, raised rash
- brain fog
- autoimmune disease symptoms
- weight gain
- anxiety/depression
- hair loss
- severe anxiety
- numbness of extremities
- joint pain
- back pain
- suicidal thoughts
Postmarketing regulatory history
A Facebook group called Essure Problems which (as of 04/03/2017) had 33,140 members called the method "E-hell" and mentioned mostly pain, bleeding, bloating and other side effects from the device. Some women had coils break and perforate their internal organs, conceived and gave birth to a newborn, at a number well above what Bayer has been reporting.[6] Erin Brockovich became involved in the controversy due to the laws of Federal preemption which stop women suing Bayer,[6] and hosts a website where women can share their stories after having the procedure.[5][6]
Since then Bayer provided two Toll-free telephone numbers for patient complaints,[6] has advised that women reporting adverse effects are "consistent with clinical trials and consistent with what the FDA is seeing",[6] and further insisted that it wanted to hear from any women experiencing problems with Essure.[6]
In April 2015, a group of 6 delegates from the Essure Problems group, including a doctor with Essure experience spoke before 36 members of the FDA and the Congressional HELP committee regarding a citizen's petition filed with the FDA. The FDA began investigating the claims of then over 16,000 members of the group as well as the legalities of the approval process that essure went through.[citation needed] As of 2015, one postmarketing study was not published for 13 years after the device was approved, and another postmarketing study had not been published as of 2015.[1]
FDA
The product was FDA approved in 2002.[6] In 2013, the product made news in North America, with women complaining of severe side effects leading to surgical extraction. According to one article, women who have gotten pregnant are naming these children e-babies.[5]
In October 2013 the FDA stated that since the product was approved in 2002 it had received 943 reports of adverse events related to Essure, mainly for pain (606 of the complaints).[6] An additional 1,000 more complaints have been sent to the FDA in a voluntary reporting system, but physicians are not obliged to report complaints.[6]
In June 2015, the FDA reported an investigation into Essure and its over 5000 complaints, 7 reported deaths, and many additional side effects, all linked to Essure, its specific chemical composition, its improper placement and its insertion. It announced, its Obstetrics and Gynecology Devices Panel would conduct an evidence-based review of Essure's safety in September 2015 due to the rise in adverse event reports from only 950 reports between 2002 through October 2013, to more than 4,150, or 81 percent of the total, from October 2013 to June 2015.[22]
End of February 2016, the FDA issued a "black box" label to warn the public about the harmful complications associated with the use of this device and requested Bayer to conduct a new postmarket surveillance to follow 2,000 women for at least three years, comparing the effectiveness and safety of the device with other surgical contraceptive methods. Women and doctors were required to sign a decision checklist before Essure implantation, and to give consent to a test three months later to ensure the device was properly placed and functioning.[23]
References
- ^ a b c "Revisiting Essure--Toward Safe and Effective Sterilization". N Engl J Med. 373 (15): e17. Oct 8, 2015. doi:10.1056/NEJMp1510514. PMID 26397951.
{{cite journal}}: Cite uses deprecated parameter|authors=(help)Free full text - ^ a b c "Essure[[Trademark|™]] System - P020014". US Food and Drug Administration. 2009-06-29. Retrieved 2011-05-21.
{{cite web}}: URL–wikilink conflict (help) - ^ "Essure Permanent Birth Control". U.S. Food and Drug Administration. Retrieved June 24, 2014.
- ^ a b Hurskainen, R.; Hovi, S.; Gissler, M.; Grahn, R.; Kukkonen-Harjula, K.; Nord-Saari, M.; Mäkelä, M. (2010). "Hysteroscopic tubal sterilization: a systematic review of the Essure system". Fertility and Sterility. 94 (1): 16–19. doi:10.1016/j.fertnstert.2009.02.080. PMID 19409549.
- ^ a b c d Deirdre Dearhoff (December 22, 2013). "Women report complications from Essure birth control". Chicago Tribune. Retrieved March 2, 2014.
- ^ a b c d e f g h i j Regan Morris (June 24, 2014). "Erin Brockovich calls for end to Bayer's Essure". BBC News, Los Angeles. Retrieved June 24, 2014.
- ^ Stein, Rob (21 September 2015). "FDA Revisits Safety Of Essure Contraceptive Device". NPR. Retrieved 21 September 2015.
- ^ a b "Safety and efficacy of hysteroscopic sterilization compared with laparoscopic sterilization: an observational cohort study". BMJ. 351: h5162. 13 Nov 2015. doi:10.1136/bmj.h5162. PMC 4604215. PMID 26462857.
{{cite journal}}: Cite uses deprecated parameter|authors=(help)Free full text - ^ Isle, Jay W. Belle (17 August 2017). "Essure Loses Commercial License in European Union - Legal Reader". Legalreader.com. Retrieved 16 May 2018.
- ^ "Essure Sales Halted In European Union, Implants Recalled". Birthcontrolproblems.com. 7 August 2017. Retrieved 16 May 2018.
- ^ "EU suspends sale of contraceptive implant". Medicalspress.com. Retrieved 16 May 2018.
- ^ "Satojen naisten sterilisaatiot menneet pieleen Suomessa – vaeltamaan lähteneitä implantteja löydetty muualta elimistöstä". Yle Uutiset. Retrieved 16 May 2018.
- ^ US FDA Medical Devices--FDA Activities: Essure Page Last updated 04/09/2018, accessed 16 May 2018
- ^ a b c d e Miño M, Arjona JE, Cordón J, Pelegrin B, Povedano B, Chacon E (June 2007). "Success rate and patient satisfaction with the Essure sterilisation in an outpatient setting: a prospective study of 857 women". BJOG. 114 (6): 763–6. doi:10.1111/j.1471-0528.2007.01354.x. PMID 17516970. Retrieved 15 December 2015.
- ^ [1] [dead link]
- ^ "Essure Confirmation Test". Conceptus Inc. Archived from the original on 2011-06-12. Retrieved 2011-05-30.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Clinical Testing". Essure. Conceptus. Retrieved 2006-12-12. [dead link]
- ^ Smith RD (January 2010). "Contemporary hysteroscopic methods for female sterilization". Int J Gynaecol Obstet. 108 (1): 79–84. doi:10.1016/j.ijgo.2009.07.026. PMID 19716128.
- ^ a b "Prescribing Information" (PDF). Essure. Conceptus. 2005-09-08. Archived from the original (PDF) on 2006-11-11. Retrieved 2006-12-12.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Doctors Confirm First Successful Essure Tubal Ligation Reversal". medical news today. 2008-10-08. Retrieved 2010-02-15., referring to Dr. William A.C. Greene Jr. and Dr. Wendell Turner at Lakeshore Surgical Center
- ^ Hurskainen R, Hovi SL, Gissler M, et al. (April 2009). "Hysteroscopic tubal sterilization: a systematic review of the Essure system". Fertil. Steril. 94 (1): 16–19. doi:10.1016/j.fertnstert.2009.02.080. PMID 19409549.
- ^ U.S., FDA. "FDA Activities". U.S. Food and Drug Administration. Retrieved 19 August 2015.
- ^ "FDA takes additional action to better understand safety of Essure, inform patients of potential risks". U.S. Food and Drug Administration. February 29, 2016. Retrieved August 21, 2016.
