Spinal cord stimulator: Difference between revisions
Undid revision 849800212 by 137.120.236.103 (talk) unsourced and full of editorializing |
|||
| Line 53: | Line 53: | ||
==See also== |
==See also== |
||
*[[Transcutaneous electrical nerve stimulation]] |
*[[Transcutaneous electrical nerve stimulation]] |
||
Reimbursement in pain centers has dropped for prior injections therapies and interventions, including but not limited to epidural steroid injections, selective nerve blocks, medial branch blocks, facet radiofrequency denervations etc. As a result of this and the push away from opioids, device manufacturers continue to press for increased use of spinal cord stimulators, peripheral nerve stimulators, and dorsal root ganglia stimulators. The FDA does not require that such devices demonstrate the same level of proof as they do for medications (i.e. three large placebo-controlled randomized trials). As a result, devices with minimal and perhaps no benefit can be FDA approved for marketing. With each stimulator costing over $20,000 USD, there is the incentive for the device manufacturers and their representatives to press for their use. Further, physicians are reimbursed well for both the trials and implantations of these devices, far more so relative to the loss of revenue from the other procedures listed above. CMS, the financial arm of Medicare, reimburses physicians not on the procedure itself, but rather on the number of leads they implant. Hence a wire with 8 leads pays double what a single wire with 4 leads costs, despite the exact same procedure being done. The number of 8 lead wires has increased in the market dramatically. Almost all literature analyzing spinal cord stimulators comes from the very societies that are mostly funded by the device manufacturers, and whose members stand to make the most money by implanting these devices. As a result, large numbers of these devices are being implanted in patients where there is little to no indication and where there will be little benefit. One need only look for data analyzing the percentage of patients with stimulators who are still on strong opioids to see that their primary benefit is to the manufacturers and the practitioners implanting them. |
|||
==References== |
==References== |
||
Revision as of 16:22, 30 July 2018
| Spinal cord stimulator | |
|---|---|
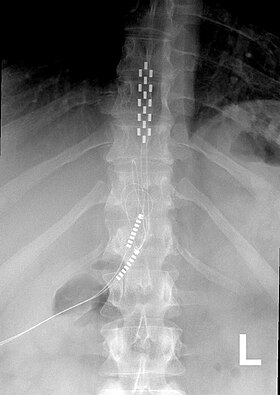 Anterior view X-ray of a spinal cord stimulator (SCS) implanted in the thoracic spine |
A Spinal Cord Stimulator (SCS) or Dorsal Column Stimulator (DCS) is a type of implantable neuromodulation device (sometimes called a "pain pacemaker") that is used to send electrical signals to select areas of the spinal cord (dorsal columns) for the treatment of certain pain conditions. SCS is a consideration for people who have a pain condition that has not responded to more conservative therapy.[1]
In the United States Failed Back Surgery Syndrome is the most common use while in Europe the most common use is peripheral ischemia.[2]
As of 2014 the FDA had approved SCS as a treatment for failed back surgery syndrome (FBSS), chronic pain, Complex Regional Pain Syndrome, intractable angina, as well as visceral abdominal and perineal pain[1] and pain in the extremities from nerve damage.[3]
Medical uses
The most common use of SCS is failed back surgery syndrome (FBSS) in the United States and peripheral ischemic pain in Europe.[4][5]
Once a person has had a psychological evaluation and deemed an appropriate candidate for SCS, a temporary implant is placed, called a trial, to determine the best stimulation pattern, and the person is sent home for three to ten days with an external pulse generator. If pain control and increased activity was achieved, a permanent system, with leads and a pulse generator, is placed.[5]
Contraindications
SCS may be contraindicated in people who have coagulation related disorders, or are on anticoagulant therapy.[1] Other contraindications include local and systemic infection, pacemakers, or those people for whom pre-surgical imaging studies show have anatomy that makes placement difficult, or if concerns arise during psychological evaluation.[6][7][8]
Adverse effects and complications
Complications with SCS range from simple easily correctable problems to devastating paralysis, nerve injury and death. In a 7-year follow-up, the overall complication rate was 5-18%. The most common complications include lead migration, lead breakage, and infection. Other complications include rotation of the pulse generator, haematomas (subcutaneous or epidural), cerebrospinal fluid (CSF) leak, post dural puncture headache, discomfort at pulse generator site, seroma and transient paraplegia.[9]
Some people find the tingling sensation caused by SPS to be unpleasant.[10]
The most common hardware related complication is lead migration, in which the implanted electrodes move from their original placement. With this complication, recapturing paraesthesia coverage can be attempted with reprogramming.[11] In circumstances involving major lead migration a reoperation may be required to reset the lead placement.[12] Studies differ greatly in reporting the percentage of people who have lead migration but the majority of studies report in the range of 10-25% of lead migration for spinal cord stimulation.[12]
Mechanism of action
The neurophysiological mechanisms of action of spinal cord stimulation are not completely understood but may involve masking pain sensation with tingling by altering the pain processing of the central nervous system.[10][13] The mechanism of analgesia when SCS is applied in neuropathic pain states may be very different from that involved in analgesia due to limb ischemia.[14][15] In neuropathic pain states, experimental evidence show that SCS alters the local neurochemistry in dorsal horn, suppressing the hyperexcitability of the neurons. Specifically, there is some evidence for increased levels of GABA release, serotonin, and perhaps suppression of levels of some excitatory amino acids, including glutamate and aspartate. In the case of ischemic pain, analgesia seems to derive from restoration of the oxygen demand supply. This effect could be mediated by inhibition of the sympathetic system, although vasodilation is another possibility. It is also probable that a combination of the two above mentioned mechanisms is involved.[16]
History
Electrotherapy of pain by neurostimulation began shortly after Melzack and Wall proposed the gate control theory in 1965. This theory proposed that nerves carrying painful peripheral stimuli and nerves carrying touch and vibratory sensation both terminate in the dorsal horn (the gate) of spinal cord.[17] It was hypothesized that input to the latter could be manipulated to “close the gate” to the former. As an application of the gate control theory, Shealy et al.[18] implanted the first spinal cord stimulator device directly on the dorsal column for the treatment of chronic pain and in 1971, Shimogi and colleagues first reported the analgesic properties of epidural spinal cord stimulation. Since then this technique has undergone numerous technical and clinical developments.
At this time neurostimulation for the treatment of pain is used with nerve stimulation, spinal cord stimulation, deep brain stimulation, and motor cortex stimulation.
Research
SCS has been studied in people with Parkinson's disease[19] and angina pectoris.[20]
Research on improving the devices and software has included efforts to increasing the battery life, efforts to develop closed loop control, and combining stimulation with implanted drug delivery systems.[19]
See also
References
- ^ a b c McKenzie-Brown, Anne Marie (November 1, 2016). "Spinal cord stimulation: Placement and management". UptoDate.
- ^ Eldabe, Sam; Kumar, Krishna; Buchser, Eric; Taylor, Rod S. (July 2010). "An analysis of the components of pain, function, and health-related quality of life in patients with failed back surgery syndrome treated with spinal cord stimulation or conventional medical management". Neuromodulation. 13 (3): 201–209. doi:10.1111/j.1525-1403.2009.00271.x. PMID 21992833.
- ^ Song, Jason J.; Popescu, Adrian; Bell, Russell L. (May 2014). "Present and potential use of spinal cord stimulation to control chronic pain". Pain Physician. 17 (3): 235–246. PMID 24850105.
- ^ Turner, J. A.; Loeser, J. D.; Bell, K. G. (December 1995). "Spinal cord stimulation for chronic low back pain: a systematic literature synthesis". Neurosurgery. 37 (6): 1088–1095, discussion 1095–1096. doi:10.1097/00006123-199512000-00008. PMID 8584149.
- ^ a b Patel, Vikram B.; Wasserman, Ronald; Imani, Farnad (2015-08-22). "Interventional Therapies for Chronic Low Back Pain: A Focused Review (Efficacy and Outcomes)". Anesthesiology and Pain Medicine. 5 (4): e29716. doi:10.5812/aapm.29716. PMC 4604560. PMID 26484298.
- ^ Narouze, Samer; Benzon, Honorio T.; Provenzano, David A.; Buvanendran, Asokumar; De Andres, José; Deer, Timothy R.; Rauck, Richard; Huntoon, Marc A. (May 2015). "Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications: guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain". Regional Anesthesia and Pain Medicine. 40 (3): 182–212. doi:10.1097/AAP.0000000000000223. PMID 25899949.
- ^ Deer, Timothy R.; Mekhail, Nagy; Provenzano, David; Pope, Jason; Krames, Elliot; Leong, Michael; Levy, Robert M.; Abejon, David; Buchser, Eric (August 2014). "The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee". Neuromodulation. 17 (6): 515–550, discussion 550. doi:10.1111/ner.12208. PMID 25112889.
- ^ Knezevic, Nebojsa N.; Candido, Kenneth D.; Rana, Shalini; Knezevic, Ivana (July 2015). "The Use of Spinal Cord Neuromodulation in the Management of HIV-Related Polyneuropathy". Pain Physician. 18 (4): E643–650. PMID 26218955.
- ^ Hayek, Salim M.; Veizi, Elias; Hanes, Michael (October 2015). "Treatment-Limiting Complications of Percutaneous Spinal Cord Stimulator Implants: A Review of Eight Years of Experience From an Academic Center Database". Neuromodulation. 18 (7): 603–608, discussion 608–609. doi:10.1111/ner.12312. PMID 26053499.
- ^ a b Deer, Timothy R.; Krames, Elliot; Mekhail, Nagy; Pope, Jason; Leong, Michael; Stanton-Hicks, Michael; Golovac, Stan; Kapural, Leo; Alo, Ken (August 2014). "The appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation Appropriateness Consensus Committee". Neuromodulation. 17 (6): 599–615, discussion 615. doi:10.1111/ner.12204. PMID 25112892.
- ^ Eldabe, Sam; Buchser, Eric; Duarte, Rui V. (2016-02-01). "Complications of Spinal Cord Stimulation and Peripheral Nerve Stimulation Techniques: A Review of the Literature". Pain Medicine. 17 (2): 325–336. doi:10.1093/pm/pnv025.
- ^ a b Kumar, Krishna; Buchser, Eric; Linderoth, Bengt; Meglio, Mario; Van Buyten, Jean-Pierre (January 2007). "Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts". Neuromodulation. 10 (1): 24–33. doi:10.1111/j.1525-1403.2007.00084.x. PMID 22151809.
- ^ Sinclair, Chantelle; Verrills, Paul; Barnard, Adele (2016-07-01). "A review of spinal cord stimulation systems for chronic pain". Journal of Pain Research. 9: 481–492. doi:10.2147/jpr.s108884.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Linderoth, B.; Foreman, R. D. (July 1999). "Physiology of spinal cord stimulation: review and update". Neuromodulation. 2 (3): 150–164. doi:10.1046/j.1525-1403.1999.00150.x. PMID 22151202.
- ^ Oakley, John C.; Prager, Joshua P. (2002-11-15). "Spinal cord stimulation: mechanisms of action". Spine. 27 (22): 2574–2583. doi:10.1097/00007632-200211150-00034. PMID 12435996.
- ^ Kunnumpurath, Sreekumar; Srinivasagopalan, Ravi; Vadivelu, Nalini (1 September 2009). "Spinal cord stimulation: principles of past, present and future practice: a review". Journal of Clinical Monitoring and Computing. 23 (5): 333–339. doi:10.1007/s10877-009-9201-0. PMID 19728120.
- ^ Kirkpatrick, Daniel R.; McEntire, Dan M.; Hambsch, Zakary J.; Kerfeld, Mitchell J.; Smith, Tyler A.; Reisbig, Mark D.; Youngblood, Charles F.; Agrawal, Devendra K. (December 2015). "Therapeutic Basis of Clinical Pain Modulation". Clinical and Translational Science. 8 (6): 848–856. doi:10.1111/cts.12282. PMC 4641846. PMID 25962969.
- ^ Shealy, C. N.; Mortimer, J. T.; Reswick, J. B. (July 1967). "Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report". Anesthesia and Analgesia. 46 (4): 489–491. doi:10.1213/00000539-196707000-00025. PMID 4952225.
- ^ a b de Andrade, Emerson Magno; Ghilardi, Maria Gabriela; Cury, Rubens Gisbert; Barbosa, Egberto Reis; Fuentes, Romulo; Teixeira, Manoel Jacobsen; Fonoff, Erich Talamoni (January 2016). "Spinal cord stimulation for Parkinson's disease: a systematic review". Neurosurgical Review. 39 (1): 27–35, discussion 35. doi:10.1007/s10143-015-0651-1. PMID 26219854.
- ^ Taylor, Rod S.; De Vries, Jessica; Buchser, Eric; Dejongste, Mike J. L. (2009-03-25). "Spinal cord stimulation in the treatment of refractory angina: systematic review and meta-analysis of randomised controlled trials". BMC Cardiovascular Disorders. 9: 13. doi:10.1186/1471-2261-9-13. PMC 2667170. PMID 19320999.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
