Atom
- For alternative meanings see atom (disambiguation).
You must add a |reason= parameter to this Cleanup template – replace it with {{Cleanup|November 2005|reason=<Fill reason here>}}, or remove the Cleanup template.
| Atom | ||||||
|---|---|---|---|---|---|---|

| ||||||
| Helium atom model Showing nucleus with two protons (blue) and two neutrons (red), orbited by two electrons (waves). | ||||||
| Classification | ||||||
| ||||||
| Properties | ||||||
|
An atom (Greek άτομον from ά: non and τομον: divisible) is a submicroscopic structure found in all ordinary matter. It is the smallest unit of an element to retain all the chemical properties of that element. The word atom originally meant a smallest possible particle of matter, not further divisible. Later, the objects that had been called atoms were found to be further divisible into smaller subatomic particles, but the word atom nonetheless continues to refer to them.
Most atoms are composed of three types of massive subatomic particles which govern their external properties:
- electrons, which have a negative charge and are the least massive of the three;
- protons, which have a positive charge and are about 1836 times more massive than electrons; and
- neutrons, which have no charge and are about 1838 times more massive than electrons.
Together, protons and neutrons form the nucleus of an atom, which is surrounded by the electrons. Protons and neutrons themselves are now thought to consist of varieties of a still smaller particle called the quark, and the electron is considered a type of lepton. Therefore in modern atomic theory, the two basic constituents of matter are the lepton and the quark of which the above three particles of the atom are composed. All particles exhibit a wave-particle duality so that the electron is better understood as a wave when drawn about a nucleus.
Atoms can differ in the number of each of the subatomic particles they contain. Atoms of the same element have the same number of protons, though the number of neutrons can differ creating isotopes. Atoms are electrostatically neutral if they have an equal number of protons and electrons. Atoms which have either gained or lost electrons are called ions.
Atoms are the fundamental building blocks of chemistry, and are conserved in chemical reactions. Atoms are able to bond into molecules and other types of chemical compounds. Molecules are made up of multiple atoms; for example, a molecule of water is a combination of 2 hydrogen and one oxygen atom.
Properties of the atom
Atom sizes
Possibly the biggest problem with measuring the atom is that we cannot see it. It is many times smaller than the wavelength that our eyes can detect in any kind of microscope. However, there are ways of projecting the atom so we can get types of images of it. These include: scanning tunneling microscopy (STM), atomic force microscopy (ATM), and nuclear magnetic resonance (NMR).
In measuring an atom, one must measure the size of the area that an electron can travel in. Electrons travel in areas called atomic orbitals. This area forms a cloud where the electron may be situated. In the helium atom above (shown in its ground state), the atomic orbital where the electron may be situated describes a sphere. But to complicate matters, the cloud or atomic orbital in which an electron can travel changes shapes depending on the energy of the electron. So some electrons travel in the shape of a dumbbell with the nucleus in the smallest space in-between. (Imagine holding two light bulbs away from each other with the metal bases touching each other, then the nucleus would be at the point where they touch and the electrons would sort of do figure eights.) There are other more complicated shapes as well. And the heavier the element, the more electrons there are and the more shapes there are for the orbitals in the atom. It therefore not only becomes more complicated to measure the size of the atom, but it becomes complicated to draw the atoms of heavier elements.
Since the electron orbitals are considered clouds, then the size of an atom is not easily defined since the places where the electron can be just gradually go to zero as the distance from the nucleus increases. For atoms that can form solid crystals, the distance between adjacent nuclei can give an estimate of the atom size. For atoms that do not form solid crystals other techniques are used, including theoretical calculations. As an example, the size of a hydrogen atom is estimated to be approximately 1.0586×10−10 m. Compare this to the size of the proton which is the only particle in the nucleus of the hydrogen atom which is approximately 10−15 m. Thus the ratio of the sizes of the hydrogen atom to its nucleus is about 100,000:1. Atoms of different elements do vary in size, but the sizes are roughly the same to within a factor of 2 or so. The reason for this is that elements with a large positive charge on the nucleus attract the electrons to the center of the atom more strongly. A basic analogy for the size of an atom is this: if an atom were the size of a baseball stadium, the nucleus would be the size of a marble.
Elements, isotopes and ions
Atoms are generally classified by their atomic number, which corresponds to the number of protons in the atom. The atomic number defines which element the atom is. For example, carbon atoms are those atoms containing six protons. All atoms with the same atomic number share a wide variety of physical properties and exhibit the same chemical behavior. The various kinds of atoms are listed in the periodic table in order of increasing atomic number.
The mass number, atomic mass number, or nucleon number of an element is the total number of protons and neutrons in an atom of that element, because each proton or neutron essentially has a mass of 1 amu. The number of neutrons in an atom has no effect on which element it is. Each element can have numerous different atoms with the same number of protons and electrons, but varying numbers of neutrons. Each has the same atomic number but a different mass number. These are called the isotopes of an element. When writing the name of an isotope, the element name is followed by the mass number. For example, carbon-14 contains 6 protons and 8 neutrons in each atom, for a total mass number of 14.
The simplest atom is the hydrogen atom, which has atomic number 1 and consists of one proton and one electron. The hydrogen isotope which also contains 1 neutron so is called deuterium or hydrogen-2; the hydrogen isotope with 2 neutrons is called tritium or hydrogen-3. Tritium is an unstable isotope which causes the atom to lose mass in a process called radioactivity. The elements in the periodic table beginning with number 86, radon, and those that follow which are all heavier have no stable isotopes and are all radioactive.
The atomic mass listed for each element in the periodic table is an average of the isotope masses found in nature, weighted by their abundance.
Although most sources state that there are 92 elements that occur naturally on earth from hydrogen up to uranium in the periodic table, it has been recently discovered that plutonium, the 94th element, also occurs naturally. Most of these elements were created through stellar nucleosynthesis and supernova nucleosynthesis. Several elements that do not occur on earth have been found to be present in stars. Elements not normally found in nature have been artificially created by nuclear bombardment, but they are usually unstable and spontaneously change into stable natural chemical elements by the processes of radioactive decay.
Atoms that have either lost or gained electrons are called atomic ions (with either positive(+) or negative charge(−), respectively). Atoms are canonically distinguished from ions by their balanced electrical charge.
Atomic spectrum
Each element in the periodic table therefore consists of an atom in a unique configuration i.e. with different amounts of protons in the nucleus. Each atom can also be described by the shapes of its atomic orbitals i.e. the cloud that the electrons form around the nucleus. There is also another way in which each element consisting of its own combination of protons and atomic orbitals is unique. And that is by its atomic spectrum. Think of a spectrum as what happens when light is passed through a prism. The light breaks up into its component colors. This happens in a rainbow. Spectroscopy studies the spectrum of each element. That is to say that each atom of each element creates its own light pattern unique to itself. Each element has its own spectral signature. Scientists can use a spectrometer to study the atoms in stars and other distant objects and due to the unique spectral lines that each element produces, we are able to tell the chemical composition of distant planets, stars and galaxies.
Electron configuration
- see main article electron configuration
The chemical behavior of atoms is largely due to interactions between electrons. Electrons of an atom remain within certain, predictable electron configurations. Electrons fall into shells based on their relative energy level. Generally, the higher the energy level of a shell, the further away it is from the nucleus. The electrons in the outermost shell, called the valence electrons, have the greatest influence on chemical behavior. Core electrons (those not in the outer shell) play a role, but it is usually in terms of a secondary effect due to screening of the positive charge in the atomic nucleus.
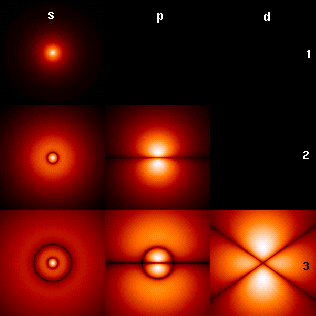
An electron shell can hold up to 2n2 electrons, where n is the number of the shell. Whichever occupied shell is currently most outward is the valence shell, even if it only has one electron. In the most stable state, an atom's electrons will fill up its shells in order of increasing energy. Under some circumstances an electron may be excited to a higher energy level (that is, it absorbs energy from an external source and leaps to a higher shell), leaving a space in a lower shell, but at some point it will fall back to its previous level, emitting its excess energy as a photon.
Electron shells also have distinctive shapes denoted by letters. In the illustration, the letters s, p, and d describe the shape of the atomic orbital. Electrons also have another interesting configuration description due to the fact that they rotate in space. Thus electrons are said to have spin (physics).
Valence and bonding
- see main article valence electrons and chemical bond
The number of electrons in an atom's outermost shell (ie the valence shell) governs its bonding behavior. Therefore, elements with the same number of valence electrons are grouped together in the periodic table of the elements. Group (i.e. column) 1 elements contain one electron on their outer shell; Group 2, two electrons; Group 3, three electrons; etc. As a general rule, the fewer electrons in an atom's valence shell, the more reactive it is. Group 1 metals are therefore very reactive, with caesium, rubidium, and francium being the most reactive of all metals.
Every atom is much more stable (i.e. less energetic) with a full valence shell. This can be achieved one of two ways: an atom can either share electrons with neighboring atoms (a covalent bond), or it can remove electrons from other atoms (an ionic bond). Another form of ionic bonding involves an atom giving some of its electrons to another atom; this also works because it can end up with a full valence by giving up its entire outer shell. By moving electrons, the two atoms become linked. This is known as chemical bonding and serves to build atoms into molecules or ionic compounds. Five major types of bonds exist:
Atoms and antimatter
- see main article antimatter
Antimatter can also form atoms, composed of antielectrons (positrons), antiprotons, and antineutrons.
Atoms and the Big Bang
In models of the Big Bang, Big Bang nucleosynthesis predicts that within one to three minutes of the Big Bang all the current atomic material in the universe was created producing no heavier element than lithium, but mostly hydrogen and helium. However, although the basic atomic particles of matter were created, atoms themselves could not form in the intense heat.
Big Bang chronology of the atom continues to approximately 379,000 years after the Big Bang when the cosmic temperature had dropped to just 3,000 K which allowed the first atoms to form. It was then cool enough to allow protons to capture one electron each and form neutral atoms of hydrogen. Hydrogen makes up approximately 75% of the atoms in the universe. Helium makes up 24% and all other elements make up 1%.
Since the size of the universe is unknown, the total numbers of atoms in the universe is unknown, but the number is not thought to be infinite because current theory suggests we live in a finite universe.
One thing we can say about the mass of the baryons in the universe, meaning the mass of the protons and neutrons, is that we can tell what the ratio of their density ought to be from the Big Bang model. Einstein's theory of General Relativity suggests that the universe is the same in all directions and from all viewpoints. Therefore, examining one region of the universe and the density of atoms in that region should tell us how densely atoms are scattered throughout the entire universe, but as said previously, does not tell us how far the universe extends and how many atoms exist in total. Big Bang Nucleosynthesis predicts that 1/20 of the total mass of the Universe is baryonic matter. (The baryon is the category used to describe neutrons and protons which are similar in mass but different in electric charge.) So theoretically we should be able to study a region of space and calculate the amount of matter we see through our telescopes and one-twentieth of the matter should be baryons. However, from the density we can see through telescopes of matter in regions of the visible universe, 99% of the baryons are missing. This has given rise to theories of dark matter (which should also be made of baryons--or if you prefer atoms, since baryons make up the nucleus of atoms) in order to make up the difference in missing matter. What that means is that there are probably more atoms out there than we can see through our usual means of detection. In other words, we cannot see visible light from these atoms nor have we detected electromagnetic radiation, but they exist. In fact, in some cases we have detected, through radio-wave detectors, entire galaxies such as Virgo H121 that do not appear in normal telescopes.
Atomic theory
The atomic theory is a theory of the nature of matter. It states that all matter is composed of atoms.
Historical theories
Democritus and Leucippus, Greek philosophers in the 5th century BC, presented the first theory of atoms (see article atomism for more details). They held that each atom had a different shape, like a pebble, that governed the atom's properties. Dalton and Avogadro rediscovered the works of Democritus and Leucippus and suggested in the 19th century that matter was made up of atoms, but they knew nothing of their structure. This theory was conflicting with the theory of infinite divisibility, which states that matter can always be divided into smaller parts. The controversy ended in 1911 when Jean Perrin demonstrated the existence of atoms through experimental validation of Einstein's theory of Brownian motion (which relied on atomic theory).
For much of this time, atoms were thought to be the smallest possible piece of matter. However, in 1897, J.J. Thomson published his work proving that cathode rays are made of negatively charged particles (electrons). Since cathode rays are essentially emitted from matter, this proved that atoms are made up of subatomic particles and are therefore divisible, and not the indivisible "atomos" Democritus talked about. Physicists later invented a new term for indivisible units, namely elementary particles since the word atom had already been taken and come into common use.
At first, it was believed that the electrons were distributed more or less uniformly in a sea of positive charge (the plum pudding model). However, an experiment conducted a few years later by Rutherford demonstrated that atoms are mostly empty space, with a lot of mass concentrated in a nucleus. In the gold foil experiment, he shot alpha particles (emitted by polonium) through a sheet of gold. He observed that most of the particles passed straight through the sheet without deflection (striking a fluorescent screen on the other side), but that, surprisingly, a small number were bounced right back (having come close to a nucleus). This led to the planetary model of the atom, in which the electrons orbited the nucleus like the planets orbiting the sun.
The nucleus was later discovered to contain protons, and further experimentation by Rutherford found that the nuclear mass of most atoms surpassed the number of protons it possessed; this led him to postulate the existence of neutrons, whose existence would be proven in 1932 by James Chadwick.
The planetary model of the atom still had shortcomings. Firstly, a moving electrical charge emits electromagnetic waves; according to classical physics, an orbiting charge would steadily lose energy and spiral towards the nucleus, eventually colliding with it. Secondly, the model didn't explain why hydrogen gas, when submitted to an electrical discharge, emitted light only in certain discrete spectra.
Experiments by Max Planck and Albert Einstein demonstrated that energy is transferred in tiny fixed amounts known as quanta. In 1913, Niels Bohr used this idea in his Bohr model of the atom, in which the electrons could only orbit the nucleus in fixed circles. They couldn't spiral downwards because they couldn't lose energy in a continuous manner; they could only make quantum leaps between fixed energy levels. The Bohr model would eventually be replaced by a full quantum mechanics model in 1925.
Study of atoms
Because of their ubiquitous nature, atoms have been an important field of study for many centuries. Current research focuses on quantum effects, such as in Bose-Einstein condensate.
The study of atoms was done by largely indirect means through the 19th century and early 20th century. In recent years, however, new techniques have made the identification and study of atoms easier and more accurate. The electron microscope, invented in 1931, can image large molecules, however, not the atom itself. Atomic force microscopy is another technique by which individual atoms can be visualized and even arranged into patterns. Methods also exist to identify atoms and compounds. Elemental analysis allows the exact identification of the types and amounts of atoms in a substance.
Practical uses of the atom
Atoms have given us the key to understanding our universe, understanding our earth and life upon it, improving technology, and creating life-saving pharmaceuticals. There does not exist a scientific field that is not affected by the understanding of the atom. Atoms are the basis for chemistry, physics, biology, astronomy and biology.
Within the tiny atom are the powers to both create and destroy. Through fusion and fission man has learned to unleash the power of the atom. Our sun and other stars use fusion of the atom to create the heavier elements in the universe that were not created in the Big Bang. Fission of the atom is used to create power in nuclear power plants. Fusion of the atom may one day be used to create safer forms of power than current fuels that are destroying the delicate balance of earth's ecosystem.
See also
- Atomism
- Chemical bond
- Exotic atom
- Individual (same literal meaning)
- Infinite divisibility
- List of particles
- Radioactive isotope
- Superatom
- Super-heavy atom
- Transuranium element
