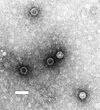
Virus quantification
"
| This is a Wikipedia user page. This is not an encyclopedia article or the talk page for an encyclopedia article. If you find this page on any site other than Wikipedia, you are viewing a mirror site. Be aware that the page may be outdated and that the user whom this page is about may have no personal affiliation with any site other than Wikipedia. The original page is located at https://en.wikipedia.org/wiki/Virus_quantification. |
"
Virus Quantification
Virus quantification involves counting the number of viruses in a specific volume to determine the virus concentration. It is utilized in both research and development (R&D) in commercial and academic laboratories as well as production situations where the quantity of virus at various steps is an important variable. For example, the production of viral vaccines, recombinant proteins using viral vectors and viral antigens all require virus quantification to continually adapt and monitor the process in order to optimize production yields and respond to ever changing demands and applications. Examples of specific instances where known viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization and adaptation of methods to cell culture. This page discusses various techniques currently used to quantify viruses in liquid samples. These methods are separated into two categories, traditional vs. modern methods. Traditional methods are industry-standard methods that have been used for decades but are generally slow and labor intensive. Modern methods are relatively new commercially available products and kits that greatly reduce quantification time. This is not meant to be an exhaustive review of all potential methods, but rather a representative cross-section of traditional methods and new, commercially available methods. While other published methods may exist for virus quantification, non-commercial methods are not discussed here.
Traditional Methods
Plaque Assay
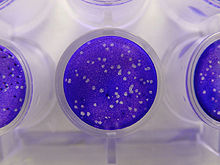
Plaque-based assays are the standard method used to determine virus concentration in terms of infectious dose. Viral plaque assays determine the number of plaque forming units (pfu) in a virus sample, which is one measure of virus quantity. This assay is based on a microbiological method conducted in petri dishes or multi-well plates. Specifically, a confluent monolayer of host cells is infected with the virus at varying dilutions and covered with a semi-solid medium, such as agar, to prevent the virus infection from spreading indiscriminately. A viral plaque is formed when a virus infects a cell within the fixed cell monolayer.[1] The virus infected cell will lyse and spread the infection to adjacent cells where the infection-to-lysis cycle is repeated. The infected cell area will create a plaque (an area of infection surrounded by uninfected cells) which can be seen visually or with an optical microscope. Plaque formation can take 3 – 14 days, depending on the virus being analyzed. Plaques are generally counted manually and the results, in combination with the dilution factor used to prepare the plate, are used to calculate the number of plaque forming units per sample unit volume (pfu/mL). The pfu/mL result represents the number of infective particles within the sample and is based on the assumption that each plaque formed is representative of one infective virus particle.[2]
50% Tissue Culture Infective Dose (TCID50)
This infectivity assay quantifies the amount of virus required to kill 50% of infected hosts or to produce a cytopathic effect (CPE) in 50% of inoculated tissue culture cells. This assay may be more common in clinical research applications where the lethal dose of virus must be determined or if the virus does not form plaques. When used in the context of tissue culture, host cells are plated and serial dilutions of the virus are added. After incubation, the percentage of cell death (i.e. infected cells) is manually observed and recorded for each virus dilution, and results are used to mathematically calculate a TCID50 result50% Tissue Culture Infective Dose (TCID50).[3] Due to distinct differences in assay methods and principles, TCID50 and pfu/mL or other infectivity assay results are not equivalent. This method can take up to a week due to cell infectivity time.[4]
Fluorescent Focus Assay (FFA)
This assay is a more rapid variation of the plaque assay because it uses immunostaining techniques with fluorescently-labeled antibodies specific for the virus antigen to measure host cell infection before an actual plaque is formed. Like the plaque assay, host cell monolayers are infected with various dilutions of the virus sample and allowed to incubate for a relatively brief incubation period (e.g., 24-72 hours). Plates are subsequently stained with fluorescent antibodies, and fluorescence microscopy is used to count and quantify how many cells are infected. The FFA method yields results in less time than plaque or TCID50 assays but is more expensive in terms of required reagents and equipment. Assay time is also dependent on the size of area that the user is counting, the larger area will take more time but will provide a more accurate representation of the sample. Results are expressed as fluorescent focus units or FFU/mL.[3]
Protein Assays
There are several variations of protein-based virus quantification assays. In general, these methods quantify either the amount of all protein or the amount of a specific virus protein in the sample rather than the number of infected cells or virus particles. Quantification most commonly relies on fluorescence detection. Some assay variations quantify protein directly in a sample while other variations require host cell infection and incubation to allow virus growth prior to protein quantification. The variation used depends primarily on the amount of protein (i.e. virus) in the initial sample and the sensitivity of the assay itself. If incubation and virus growth are required, cell and/or virus lysis/digestion are often conducted prior to analysis. Most protein-based methods are relatively fast and sensitive but require quality standards for accurate calibration, and quantify protein, not actual virus particle concentrations. Below are specific examples of widely used protein-based assays.
The hemagglutination assay (HA) is a common non-fluorescence protein quantification assay specific for influenza. It relies on the fact that hemagglutinin, a surface protein of influenza viruses, agglutinates red blood cells (i.e. causes red blood cells to clump together). In this assay, dilutions of an influenza sample are incubated with a 1% erythrocyte solution for one hour and the virus dilution at which agglutination first occurs is visually determined. The assay produces a result of hemagglutination units (HAU), with typical pfu to HAU ratios in the 106 range.[5][6][7] This assay takes ~1-2 hours to complete and results can differ widely based on the technical expertise of the operator. The hemagglutination inhibition assay is a common variation of the HA assay used to measure flu-specific antibody levels in blood serum. In this variation, serum antibodies to the influenza virus will interfere with the virus attachment to red blood cells. Therefore hemagglutination is inhibited when antibodies are present at a sufficient concentration.[8]
The BCA (bicinchoninic acid) assay is based on a simple colorimetric measurement and is the most common protein quantification assay. BCA is similar to the Lowry or Bradford protein assays and was first made commercially available by Pierce, which is now owned by Thermo Fisher Scientific. In the BCA assay, a protein’s peptide bonds quantitatively reduce Cu2+ to Cu1+, which produces a light blue color. BCA chelates Cu1+ at a 2:1 ratio resulting in a more intensely colored species that absorbs at 562 nm. Absorbance of a sample at 562 nm is used to determine the bulk protein concentration in the sample. Assay results are compared with known standard curves after analysis with a spectrophotometer or plate reader.[9] Total assay time is 30 minutes to one hour. While this assay is ubiquitous and fast, it lacks specificity.
Single radial immunodiffusion assay (SRID), also known as the Mancini method, is a protein assay that detects the amount of specific viral antigen by immunodiffusion in a semi-solid medium (e.g., agar). The medium contains antiserum specific to the antigen of interest and the antigen is placed in the center of the disc. As the antigen diffuses into the medium it creates a precipitate ring that grows until equilibrium is reached. Assay time can range from 10 hours to days depending on equilibration time of the antigen and antibody. The zone diameter from the ring is linearly related to the log of protein concentration and is compared to zone diameters for known protein standards for quantification.[10] There are kits and serums commercially available for this assay (e.g. The Binding Site Inc.).
Transmission Electron Microscopy (TEM)
TEM is a specialized type of microscopy that utilizes a beam of electrons focused with a magnetic field to image a sample. TEM provides imaging with 1000x greater spatial resolution than a light microscope (resolution down to 0.2 nm).[11] An ultrathin, negatively stained sample is required. Sample preparations involve depositing specimens onto a coated TEM grid and negative staining with an electron-opaque liquid.[12] Tissue embedded samples can also be examined if thinly sectioned. Sample preparations vary depending on protocol and user but generally require hours to complete. TEM images can show individual virus particles and quantitative image analysis can be used to determine virus concentrations. These high resolution images also provide particle morphology information that most other methods cannot. Quantitative TEM results will often be greater than results from other assays as all particles, regardless of infectivity, are quantified in the reported virus-like particles per mL (vlp/mL) result. Quantitative TEM generally works well for virus concentrations greater than 106 particles/mL. Because of high instrument cost and the amount of space and support facilities needed, TEM equipment is available in a limited number of facilities.
Modern Methods
Flow Cytometry
While most flow cytometers do not have sufficient sensitivity, there are a few commercially available instruments that can be used for virus quantification. For example, the Virus Counter® is a benchtop flow cytometer designed specifically for virus quantification by InDevR Inc[13]. This system is significantly more sensitive than other commercial cytometers, which enables efficient detection and accurate quantification of viruses while maintaining the speed and versatility advantages of flow cytometry. The Virus Counter assay and instrument quantify the number of intact virus particles in a sample using fluorescence to detect colocalized proteins and nucleic acids. Samples are stained with two dyes, one specific for proteins and one specific for nucleic acids, and analyzed as they flow through a laser beam. The quantity of particles producing simultaneous events on each of the two distinct fluorescence channels is determined, along with the measured sample flow rate, to calculate a concentration of virus-like particles (vlp/mL).[14] The results are generally similar in absolute quantity to a TEM result. The assay has a linear working range of 105-109 vlp/mL and an analysis time of ~10 min with a short sample preparation time.
Quantitative Polymerase Chain Reaction (qPCR)

Quantitative PCR utilizes polymerase chain reaction chemistry to amplify viral DNA or RNA to produce high enough concentrations for detection and quantification by fluorescence. In general, quantification by qPCR relies on serial dilutions of standards of known concentration being analyzed in parallel with the unknown samples for calibration and reference. Quantitative detection can be achieved using a wide variety of fluorescence detection strategies, including sequence specific probes or universal probes such as SYBR Green dye.[15] Sequence specific probes, such as TaqMan® (i.e. Applied Biosystems), Molecular Beacons, or Scorpion®, bind only to the copied, or cDNA of appropriate sequence produced during the reaction. SYBR Green dye binds to all double-stranded DNA[16] produced during the reaction. While SYBR Green is easy to use, its lack of specificity and lower sensitivity lead most labs to use probe-based qPCR detection schemes. There are many variations of qPCR including the comparative threshold method, which allows relative quantification through comparison of Ct values (PCR cycles that show statistically significant increases in the product) from multiple samples that include an internal standard.[17] Since PCR amplifies all target nucleic acid, whether from an intact virion or free nucleic acids in solution, qPCR results (expressed in terms of genome copies/mL) are likely to be higher in quantity than TEM results. Commercially available products for qPCR are available through numerous companies such as Invitrogen, Roche and Qiagen just to name a few. Real-time qPCR takes around 1-4 hours and can provide quantitative results containing too few viruses to be analyzed by other methods.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA is a more modern variation of a protein assay that utilizes a specific antibody linked to an enzyme to detect the presence of an unknown amount of antigen (i.e. virus) in a sample. The antibody-antigen binding event is detected and/or quantified through the enzyme’s ability to convert a reagent to a detectable signal that can be used to calculate the concentration of the antigen in the sample. Horseradish peroxidase (HRP) is a common enzyme utilized in ELISA schemes due to its ability to amplify signal and increase assay sensitivity. There are many variations, or types of ELISA assays but they can generally be classified as either indirect, competitive, sandwich or reverse. ELISA kits are commercially available from numerous commercial sources and quantification generally occurs via chromogenic reporters or fluorescence (e.g. Invitrogen, Santa Cruz Biotechnology Inc.). This technique is much less labor intensive than the traditional methods and can take anywhere from 4-24 hours based on antibody incubation time.
References
- ^ Kaufmann, S.H. (2002). Methods in Microbiology Vol.32:Immunology of Infection. Academic Press.
{{cite book}}: Text "isbn=052121678" ignored (help) - ^ Martin, S.J. (1978). The Biochemistry of Viruses. Cambridge University Press.
{{cite book}}: Text "isbn=012402033X" ignored (help) - ^ a b Flint, S.J. (2009). "Virological Methods". Principles of Virology. ASM Press.
{{cite encyclopedia}}: Text "isbn=1555814433" ignored (help) - ^ http://www.urmc.rochester.edu/mbi/resources/Xenopus/protocols/TCID50-protocol.pdf
- ^ Killian, M.L. (2008). "Hemagglutination Assay for the Avian Influenza Virus". In Spackman, Erica (ed.). Avian Influenza Virus. Vol. 436. Humana Press. pp. 47–52.
{{cite encyclopedia}}: Cite has empty unknown parameter:|1=(help); Text "isbn=9781588299390" ignored (help) - ^ Rimmelzwaan, G.F.; Baars, M.; Claas, E.C.J.; Osterhaus, A.D.M.E. (1998). "Comparison of RNA Hybridization, Hemaaglutination Assay, Titration of Infectious Virus and Immunofluorescence as Methods for Monitoring Influenza Virus Replication In Vitro". Journal of Virological Methods. 74: 57–66.
- ^ Kato, A.; Kiyotani, K.; Sakai, Y.; Yoshida, T.; Nagai, Y. (1997). "The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis". The EMBO Journal. 16: 578–587.
- ^ http://www.virology.ws/2009/05/27/influenza-hemagglutination-inhibition-assay/
- ^ http://www.piercenet.com/products/browse.cfm?fldID=02020101
- ^ Rodda, S.J.; Gallichio, H.A.; Hampson, A.W (1981). "The Single Radial Immunodiffusion Assay Highlights Small Antigenic Differences Among Influenza Virus Hemagglutinins". Journal of Clinical Microbiology. 14 (5): 479–482.
- ^ Sherman, I. "Resolution of an Electron Microscope". The Physics Factbook. Retrieved February 25 2010.
{{cite web}}: Check date values in:|accessdate=(help); Cite has empty unknown parameter:|1=(help) - ^ Steffens, W.L. (1998). "Use of Transmission Electron Microscopy for Viral Diagnosis in Psittacine Birds". Proceedings of International Virtual Conferences in Veterinary Medicine: Diseases of Psittacine Birds. Athens, Georgia.
{{cite conference}}: Unknown parameter|booktitle=ignored (|book-title=suggested) (help) - ^ InDevR:Virus Counter
- ^ Stoffel, C.L.; Finch, R.; Christensen, K.; Edwards, D.; Rowlen, K.L. (2005). "Rapid Determination of Baculovirus Titer by a Dual Channel Virus Counter". American Biotechnology Laboratory. 37 (22): 24–25.
- ^ http://www.protocol-online.org/prot/Molecular_Biology/PCR/Real-Time_PCR/index.html
- ^ http://www.appliedbiosystems.com/support/tutorials/pdf/rtpcr_vs_tradpcr.pdf
- ^ O'Leary, J.J.; Sheils, O.; Martin, C.; Crowley, A. (2003). "Taqman Technology and Real-Time Polymerase Chain Reaction". In Crocker, J.; Murray, P.G. (eds.). Molecular BIology in Cellular Pathology. John Wiley and Sons. pp. 251–268.
{{cite encyclopedia}}: Cite has empty unknown parameter:|1=(help); Text "isbn=9780470844755" ignored (help)