Binding energy
| Nuclear physics |
|---|
 |
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system has typically a lower potential energy than its constituent parts; this is what keeps the system together. The usual convention is that this corresponds to a positive binding energy.
In general, binding energy represents the mechanical work which must be done in acting against the forces which hold an object together, while disassembling the object into component parts separated by sufficient distance that further separation requires negligible additional work.
At the atomic level the atomic binding energy of the atom derives from electromagnetic interaction and is the energy required to disassemble an atom into free electrons and a nucleus.
Electron binding energy is a measure of the energy required to free electrons from their atomic orbits.
At the nuclear level the nuclear binding energy (binding energy of nucleons into a nuclide) is derived from the strong nuclear force and is the energy required to disassemble a nucleus into the same number of free unbound neutrons and protons it is composed of, in such a way that the particles are far/distant enough from each other so that the strong nuclear force can no longer cause the particles to interact.
In astrophysics, gravitational binding energy of a celestial body is the energy required to expand the material to infinity. This quantity is not to be confused with the gravitational potential energy, which is the energy required to separate two bodies, such as a celestial body and a satellite, to infinite distance, keeping each intact (the latter energy is lower).
In bound systems, if the binding energy is removed from the system, it must be subtracted from the mass of the unbound system, simply because this energy has mass, and if subtracted from the system at the time it is bound, will result in removal of mass from the system. System mass is not conserved in this process because the system is not closed during the binding process.
Mass deficit
Classically a bound system is at a lower energy level than its unbound constituents, its mass must be less than the total mass of its unbound constituents. For systems with low binding energies, this "lost" mass after binding may be fractionally small. For systems with high binding energies, however, the missing mass may be an easily measurable fraction.
Since all forms of energy have mass, the question of where the missing mass of the binding energy goes, is of interest. The answer is that this mass is lost from a system which is not closed. It transforms to heat, light, higher energy states of the nucleus/atom or other forms of energy, but these types of energy also have mass, and it is necessary that they be removed from the system before its mass may decrease. The "mass deficit" from binding energy is therefore removed mass that corresponds with removed energy, according to Einstein's equation E = mc2. Once the system cools to normal temperatures and returns to ground states in terms of energy levels, there is less mass remaining in the system than there was when it first combined and was at high energy. Mass measurements are almost always made at low temperatures with systems in ground states, and this difference between the mass of a system and the sum of the masses of its isolated parts is called a mass deficit. Thus, if binding energy mass is transformed into heat, the system must be cooled (the heat removed) before the mass-deficit appears in the cooled system. In that case, the removed heat represents exactly the mass "deficit", and the heat itself retains the mass which was lost.[1]
As an illustration, consider two objects attracting each other in space through their gravitational field. The attraction force accelerates the objects and they gain some speed toward each other converting the potential (gravity) energy into kinetic (movement) energy. When either the particles 1) pass through each other without interaction or 2) elastically repel during the collision, the gained kinetic energy (related to speed), starts to revert into potential form driving the collided particles apart. The decelerating particles will return to the initial distance and beyond into infinity or stop and repeat the collision (oscillation takes place). This shows that the system, which loses no energy, does not combine (bind) into a solid object, parts of which oscillate at short distances. Therefore, in order to bind the particles, the kinetic energy gained due to the attraction must be dissipated (by resistive force). Complex objects in collision ordinarily undergo inelastic collision, transforming some kinetic energy into internal energy (heat content, which is atomic movement), which is further radiated in the form of photons—the light and heat. Once the energy to escape the gravity is dissipated in the collision, the parts will oscillate at closer, possibly atomic, distance, thus looking like one solid object. This lost energy, necessary to overcome the potential barrier in order to separate the objects, is the binding energy. If this binding energy were retained in the system as heat, its mass would not decrease. However, binding energy lost from the system (as heat radiation) would itself have mass, and directly represent of the "mass deficit" of the cold, bound system.
Closely analogous considerations apply in chemical and nuclear considerations. Exothermic chemical reactions in closed systems do not change mass, but (in theory) become less massive once the heat of reaction is removed. This mass change is too small to measure with standard equipment. In nuclear reactions, however, the fraction of mass that may be removed as light or heat, i.e., binding energy, is often a much larger fraction of the system mass. It may thus be measured directly as a mass difference between rest masses of reactants and products. This is because nuclear forces are comparatively stronger than Coulombic forces associated with the interactions between electrons and protons that generate heat in chemistry.
Mass defect
In simple words, the definition of mass defect can be stated as follows:
Definition: The difference between the unbound system calculated mass and experimentally measured mass of nucleus is called mass defect. It is denoted by Δm. It can be calculated as follows:
- Mass defect = (unbound system calculated mass) - (measured mass of nucleus)
- i.e, (sum of masses of protons and neutrons) - (measured mass of nucleus)
In nuclear reactions, the energy that must be radiated or otherwise removed as binding energy may be in the form of electromagnetic waves, such as gamma radiation, or as heat. Again, however, no mass deficit can in theory appear until this radiation has been emitted and is no longer part of the system.
The energy given off during either nuclear fusion or nuclear fission is the difference between the binding energies of the fuel and the fusion or fission products. In practice, this energy may also be calculated from the substantial mass differences between the fuel and products, once evolved heat and radiation have been removed.
When the nucleons are grouped together to form a nucleus, they lose a small amount of mass, i.e., there is mass defect. This mass defect is released as (often radiant) energy according to the relation E = mc2; thus binding energy = mass defect × c2.
This energy is a measure of the forces that hold the nucleons together, and it represents energy which must be supplied from the environment if the nucleus is to be broken up. It is known as binding energy, and the mass defect is a measure of the binding energy because it simply represents the mass of the energy which has been lost to the environment after binding.
Mass excess
It is observed experimentally that the mass of the nucleus is smaller than the number of nucleons each counted with a mass of 1 a.m.u.. This difference is called mass excess.
The difference between the actual mass of the nucleus measured in atomic mass units and the number of nucleons is called mass excess i.e.
Mass excess = M/A = Excess-energy / c2
with : M equals the actual mass of the proton, in u.
and : A equals the mass caballo.
This mass excess is a practical value calculated from experimentally measured nucleon masses and stored in nuclear databases. For middle-weight nuclides this value is negative in contrast to the mass defect which is never negative for any nuclide.
Nuclear binding energy
Practice: Binding energy for atoms
The amount of energy required to break the nucleus of an atom into its isolated nucleons is called nuclear binding energy. The measured mass deficits of isotopes are always listed as mass deficits of the neutral atoms of that isotope, and mostly in MeV. As a consequence, the listed mass deficits are not a measure for the stability or binding energy of isolated nuclei, but for the whole atoms. This has very practical reasons, because it is very hard to totally ionize heavy elements, i.e. strip them of all of their electrons.
This practice is useful for other reasons, too: Stripping all the electrons from a heavy unstable nucleus (thus producing a bare nucleus) will change the lifetime of the nucleus, indicating that the nucleus cannot be treated independently (Experiments at the heavy ion accelerator GSI). This is also evident from phenomena like electron capture. Theoretically, in orbital models of heavy atoms, the electron orbits partially inside the nucleus (it doesn't orbit in a strict sense, but has a non-vanishing probability of being located inside the nucleus).
Of course, a nuclear decay happens to the nucleus, meaning that properties ascribed to the nucleus will change in the event. But for the following considerations and examples, you should keep in mind that "mass deficit" as a measure for "binding energy", and as listed in nuclear data tables, means "mass deficit of the neutral atom" and is a measure for stability of the whole atom.
Nuclear binding energy curve
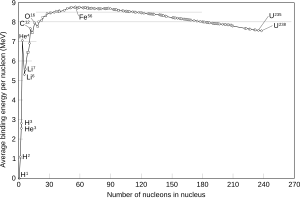
In the periodic table of elements, the series of light elements from hydrogen up to sodium is observed to exhibit generally increasing binding energy per nucleon as the atomic mass increases. This increase is generated by increasing forces per nucleon in the nucleus, as each additional nucleon is attracted by all of the other nucleons, and thus more tightly bound to the whole.
The region of increasing binding energy is followed by a region of relative stability (saturation) in the sequence from magnesium through xenon. In this region, the nucleus has become large enough that nuclear forces no longer completely extend efficiently across its width. Attractive nuclear forces in this region, as atomic mass increases, are nearly balanced by repellent electromagnetic forces between protons, as the atomic number increases.
Finally, in elements heavier than xenon, there is a decrease in binding energy per nucleon as atomic number increases. In this region of nuclear size, electromagnetic repulsive forces are beginning to gain against the strong nuclear force.
At the peak of binding energy, nickel-62 is the most tightly-bound nucleus (per nucleon), followed by iron-58 and iron-56.[2] This is the approximate basic reason why iron and nickel are very common metals in planetary cores, since they are produced profusely as end products in supernovae and in the final stages of silicon burning in stars. However, it is not binding energy per defined nucleon (as defined above) which controls which exact nuclei are made, because within stars, neutrons are free to convert to protons to release even more energy, per generic nucleon, if the result is a stable nucleus with a larger fraction of protons. Thus, iron-56 has the most binding energy of any group of 56 nucleons (because of its relatively larger fraction of protons), even while having less binding energy per nucleon than nickel-62, if this binding energy is computed by comparing Ni-62 with its disassembly products of 28 protons and 34 neutrons. In fact, it has been argued that photodisintegration of 62Ni to form 56Fe may be energetically possible in an extremely hot star core, due to this beta decay conversion of neutrons to protons.[3]
It is generally believed that iron-56 is more common than nickel isotopes in the universe for mechanistic reasons, because its unstable progenitor nickel-56 is copiously made by staged build-up of 14 helium nuclei inside supernovas, where it has no time to decay to iron before being released into the interstellar medium in a matter of a few minutes as a star explodes. However, nickel-56 then decays to iron-56 within a few weeks. The gamma ray light curve of such a process has been observed to happen in type IIa supernovae, such as SN1987a. In a star, there are no good ways to create nickel-62 by alpha-addition processes, or else there would presumably be more of this highly-stable nuclide in the universe.
Measuring the binding energy
The existence of a maximum in binding energy in medium-sized nuclei is a consequence of the trade-off in the effects of two opposing forces which have different range characteristics. The attractive nuclear force (strong nuclear force), which binds protons and neutrons equally to each other, has a limited range due to a rapid exponential decrease in this force with distance. However, the repelling electromagnetic force, which acts between protons to force nuclei apart, falls off with distance much more slowly (as the inverse square of distance). For nuclei larger than about four nucleons in diameter, the additional repelling force of additional protons more than offsets any binding energy which results between further added nucleons as a result of additional strong force interactions; such nuclei become less and less tightly bound as their size increases, though most of them are still stable. Finally, nuclei containing more than 209 nucleons (larger than about 6 nucleons in diameter) are all too large to be stable, and are subject to spontaneous decay to smaller nuclei.
Nuclear fusion produces energy by combining the very lightest elements into more tightly-bound elements (such as hydrogen into helium), and nuclear fission produces energy by splitting the heaviest elements (such as uranium and plutonium) into more tightly-bound elements (such as barium and krypton). Both processes produce energy, because middle-sized nuclei are the most tightly bound of all.
As seen above in the example of deuterium, nuclear binding energies are large enough that they may be easily measured as fractional mass deficits, according to the equivalence of mass and energy. The atomic binding energy is simply the amount of energy (and mass) released, when a collection of free nucleons are joined together to form a nucleus.
Nuclear binding energy can be easily computed from the easily measurable difference in mass of a nucleus, and the sum of the masses of the number of free neutrons and protons that make up the nucleus. Once this mass difference, called the mass defect or mass deficiency, is known, Einstein's mass-energy equivalence formula E = mc² can be used to compute the binding energy of any nucleus. (As a historical note, early nuclear physicists used to refer to computing this value as a "packing fraction" calculation.)
For example, the atomic mass unit (1 u) is defined to be 1/12 of the mass of a 12C atom—but the atomic mass of a 1H atom (which is a proton plus electron) is 1.007825 u, so each nucleon in 12C has lost, on average, about 0.8% of its mass in the form of binding energy.
Semiempirical formula for nuclear binding energy
For a nucleus with A nucleons, including Z protons and N neutrons, a semiemipirical formula for the binding energy (B.E.) per nucleon (A) is:
where the binding energy is in MeV for the following numerical values of the constants: ; ; ; ; .
The first term is called the saturation contribution and ensures that the B.E. per nucleon is the same for all nuclei to a first approximation. The term is a surface tension effect and is proportional to the number of nucleons that are situated on the nuclear surface; it is largest for light nuclei. The term is the Coulomb electrostatic repulsion; this becomes more important as increases. The symmetry correction term takes into account the fact that in the absence of other effects the most stable arrangement has equal numbers of protons and neutrons; this is because the n-p interaction in a nucleus is stronger than either the n-n or p-p interaction. The pairing term is purely empirical; it is + for even-even nuclei and - for odd-odd nuclei.
Example values deduced from experimentally measured atom nuclide masses
All mass excess data are taken from [4]. Notice also that we use 1 u = 1 a.m.u = 931.494028(±0.000023) MeV. To calculate the "binding energy" we use the formula P*(mp+me) + N * mn - mnuclide where P denotes the number of protons of the nuclides and N its number of neutrons. We take mp = 938.2723 Mev, me = 0.5110 MeV and mn = 939.5656 MeV. The letter A denotes the sum of P and N (number of nucleons in the nuclide). If we assume the reference nucleon has the mass of a neutron (so that all "total" binding energies calculated are maximal) we could define the total binding energy as the difference from the mass of the nucleus, and the mass of a collection of A free neutrons. In other words, it would be [(P+N)* mn] - mnuclide. The "total binding energy per nucleon" would be this value divided by A.
| nuclide | P | N | mass excess | total mass | total mass / A | total binding energy / A | mass defect | binding energy | binding energy / A |
|---|---|---|---|---|---|---|---|---|---|
| 56Fe | 26 | 30 | -60.6054 MeV | 55.934937 u | 0.9988382 u | 9.1538 MeV | 0.528479 u | 492.275 MeV | 8.7906 MeV |
| 58Fe | 26 | 32 | -62.1534 MeV | 57.933276 u | 0.9988496 u | 9.1432 MeV | 0.547471 u | 509.966 MeV | 8.7925 MeV |
| 60Ni | 28 | 32 | -64.4721 MeV | 59.930786 u | 0.9988464 u | 9.1462 MeV | 0.565612 u | 526.864 MeV | 8.7811 MeV |
| 62Ni | 28 | 34 | -66.7461 MeV | 61.928345 u | 0.9988443 u | 9.1481 MeV | 0.585383 u | 545.281 MeV | 8.7948 MeV |
In this calculation 56Fe has the lowest nucleon-specific mass of the four nuclides, but this does not mean it is the strongest bound atom per hadron, unless the choice of beginning hadrons is completely free. Iron releases the largest energy if any 56 nucleons are allowed to build a nuclide—changing one to another if necessary, The highest "binding energy" per hadron, with the hadrons starting as the same number of protons Z and total nucleons A as in the bound nucleus, is 62Ni. Thus, the true absolute value of the total binding energy of a nucleus depends on what we are "allowed" to construct the nucleus out of. If all nuclei of mass number A were to be allowed to be constructed of A neutrons, then Fe-56 would release the most energy per nucleon, since it has a larger fraction of protons than Ni-62. However, if nucleons are required to be constructed of only the same number of protons and neutrons that they contain, then nickel-62 is the most tightly bound nucleus, per nucleon.
| nuclide | P | N | mass excess | total mass | total mass / A | total binding energy / A | mass defect | binding energy | binding energy / A |
|---|---|---|---|---|---|---|---|---|---|
| n | 0 | 1 | 8.0716 MeV | 1.008665 u | 1.008665 u | 0.0000 MeV | 0 u | 0 MeV | 0 MeV |
| 1H | 1 | 0 | 7.2890 MeV | 1.007825 u | 1.007825 u | 0.7826 MeV | 0.0000000146 u | 0.0000136 MeV | 13.6 eV |
| 2H | 1 | 1 | 13.13572 MeV | 2.014102 u | 1.007051 u | 1.50346 MeV | 0.002388 u | 2.22452 MeV | 1.11226 MeV |
| 3H | 1 | 2 | 14.9498 MeV | 3.016049 u | 1.005350 u | 3.08815 MeV | 0.0091058 u | 8.4820 MeV | 2.8273 MeV |
| 3He | 2 | 1 | 14.9312 MeV | 3.016029 u | 1.005343 u | 3.09433 MeV | 0.0082857 u | 7.7181 MeV | 2.5727 MeV |
In the table above it can be seen that the decay of a neutron, as well as the transformation of tritium into helium-3, releases energy; hence, it manifests a stronger bound new state when measured against the mass of an equal number of neutrons (and also a lighter state per number of total hadrons). Such reactions are not driven by changes in binding energies as calculated from perviously fixed N and Z numbers of neutrons and protons, but rather in decreases in the total mass of the nuclide/per nucleon, with the reaction.
References
- ^ E. F. Taylor and J. A. Wheeler, Spacetime Physics, W.H. Freeman and Co., NY. 1992. ISBN 0-7167-2327-1, see pp. 248-9 for discussion of mass remaining constant after detonation of nuclear bombs, until heat is allowed to escape.
- ^ Fewell, M. P. (1995). "The atomic nuclide with the highest mean binding energy". American Journal of Physics. 63 (7): 653–658. doi:10.1119/1.17828.
- ^ M.P. Fewell, 1995
- ^ Jagdish K. Tuli, Nuclear Wallet Cards, 7th edition, April 2005, Brookhaven National Laboratory, US National Nuclear Data Center
External links
- Graph of Binding Energies of the elements
- Investigating Binding Energies and Mass Defect (Excel)
- Nuclear Binding energy
- Mass and Nuclide Stability
- Experimental atomic mass data compiled Nov. 2003












