Monensin
| |
| Names | |
|---|---|
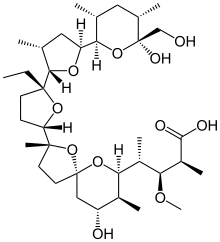
| IUPAC name
4-[2-[5-ethyl-5-[5-[6-hydroxy-6-
(hydroxymethyl)-3,5-dimethyl-oxan-2-yl]- 3-methyl-oxolan-2-yl]oxolan-2-yl]- 9-hydroxy-2,8-dimethyl-1,6-dioxasp iro[4.5]dec-7-yl]-3-methoxy-2-methyl- pentanoic acid | |
| Other names
monensic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.398 |
| E number | E714 (antibiotics) |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C36H62O11 | |
| Molar mass | 670.871g/mol |
| Appearance | solid state, white crystals |
| Melting point | 104°C |
| 3x10-6 g/dm3 (20 °C) | |
| Solubility | ethanol, acetone, diethyl ether, benzene |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Monensin, isolated from Streptomyces cinnamonensis, is a well-known representative of naturally polyether ionophore antibiotics.
History
The structure of monensin was first described by Agtarap et al. in 1967, and was the first polyether antibiotic to have its structure elucidated in this way. The first total synthesis of monensin was reported in 1979 by Kishi et al.[1]
Mechanism of action
Monensin A exhibits significant preference to form complexes with monovalent cations such as: Li+, Na+, K+, Rb+, Ag+ and Tl+ [2][3][4][5]. Monensin A is able to transport these cations across lipid membranes of cells, playing an important role as an Na+/H+ antiporter. It blocks intracellular protein transport, and exhibits antibiotic, antimalarial, and other biological activities [6]. The antibacterial properties of monensin and its derivatives are a result of their ability to transport metal cations through cellular and subcellular membranes [7][8][9].

Uses
Monensin is used extensively in the beef and dairy industries to prevent coccidiosis, increase the production of propionic acid and prevent bloat.[10]. Furthermore monensin, but also its derivatives monensin methyl ester (MME), and particularly monensin decyl ester (MDE) are widely used in ion selective electrodes [11][12][13].
References
- ^ Nicolaou, K. C. (1996). Classics in Total Synthesis. Weinheim, Germany: VCH. pp. 185–187. ISBN 3-527-29284-5.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ A. Huczyński, M. Ratajczak-Sitarz, A. Katrusiak, B. Brzezinski, ”Molecular structure of the 1:1 inclusion complex of Monensin A lithium salt with acetonitrile”, J. Mol. Struct., 2007, 871, 92-97, doi:10.1016/j.molstruc.2006.07.046
- ^ A. Huczyński, M. Ratajczak-Sitarz, A. Katrusiak, B. Brzezinski ”Molecular structure of the 1:1 inclusion complex of Monensin A sodium salt with acetonitrile” J. Mol. Struct., 2007, 832, 84-89, doi:10.1016/j.molstruc.2006.07.043
- ^ A. Huczyński, M. Ratajczak-Sitarz, A. Katrusiak, B. Brzezinski, "Molecular structure of rubidium six-coordinated dihydrate complex with monensin A", J. Mol. Struct.doi:10.1016/j.molstruc.2007.12.005
- ^ M. Pinkerton, L. K. Steinrauf, "Molecular structure of monovalent metal cation complexes of monensin", J. Mol. Biol., 1970 49(3), 533-546
- ^ H. H. Mollenhauer, D. J. Morre, L. D. Rowe, ”Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity”, Biochim. Biophys. Acta, 1990, 1031(2), 225-246, doi:10.1016/0304-4157(90)90008-Z
- ^ A. Huczyński, J. Stefańska, P. Przybylski, B. Brzezinski and F. Bartl, "Synthesis and antimicrobial properties of Monensin A esters", Bioorganic & Medicinal Chemistry Letters, 2008, 18, 2585-2589, doi:10.1016/j.bmcl.2008.03.038
- ^ A. Huczyński, P. Przybylski, B. Brzezinski, F. Bartl, ”Spectroscopic and semiempirical studies of a proton channel formed by the methyl ester of Monensin A”, J. Phys. Chem. B, 2006, 110, 15615-15623, doi:10.1021/jp062160o
- ^ A. Huczyński, A. Domańska, I. Paluch, J. Stefańska, B. Brzezinski, F. Bartl, "Synthesis of new semi-synthetic dipodands and tripodands from naturally occurring polyether ionophores", Tetrahedron Letters, 2008, 49(39), 5572-5575 doi:10.1016/j.tetlet.2008.06.116
- ^ T. Matsuoka, M.N. Novilla, T.D. Thomson and A.L. Donoho, "Review of monensin toxicosis in horses", J. Equine Veterinary Science, 16, 1996, 8-15, doi:10.1016/S0737-0806(96)80059-1
- ^ K. Tohda, K. Suzuki, N. Kosuge, H. Nagashima, H. Inoue K. Watanabe, ”A Sodium Ion Selective Electrode Based on a Highly Lipophilic Monensin Derivative and Its Application to the Measurement of Sodium Ion Concentrations in Serum”, Analytical Sciences, 6, 1990, 227-232, doi:10.2116/analsci.6.227
- ^ N. Kim, K. Park, I. Park, Y. Cho, Y. Bae, ”Application of a taste evaluation system to the monitoring of Kimchi fermentation”, Biosensors and Bioelectronics, 20, 2005, 2283-2291,doi:10.1016/j.bios.2004.10.007
- ^ K. Toko, ”Taste Sensor”, Sensors and Actuators B: Chemical, 64, 2000, 205-215, doi:10.1016/S0925-4005(99)00508-0
