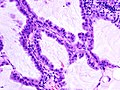
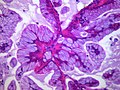
Adenocarcinoma in situ of the lung
| Adenocarcinoma in situ of the lung | |
|---|---|
| Specialty | Oncology |
Bronchioloalveolar carcinoma (BAC) is a term describing certain variants of lung cancer arising in the distal bronchioles or alveoli that initially exhibit a specific non-invasive growth pattern.
Classification

Lung cancers are an extremely heterogeneous family of malignant neoplasms,[1] with well over 50 different histological variants recognized under the 2004 revision of the World Health Organization ("WHO-2004") typing system, currently the most widely used lung cancer classification scheme.[2] Because these variants can have widely differing genetic, biological, and clinical properties, including response to treatment, correct classification of lung cancer cases are necessary to assure that lung cancer patients receive optimum management.[3][4]
Approximately 98% of lung cancers are carcinoma, which are tumors composed of cells with epithelial characteristics.[5] 8 major groups of lung carcinomas are recognized in WHO-2004:[2]
- Squamous Cell Carcinoma
- Small Cell Carcinoma
- Adenocarcinoma
- Large Cell Carcinoma
- Adenosquamous Carcinoma
- Sarcomatoid Carcinoma
- Carcinoid Tumor
- Salivary Gland-like Carcinoma
In WHO-2004, BACs are one of four specific histologic subtypes of lung adenocarcinoma, along with acinar adenocarcinoma, papillary adenocarcinoma, and solid adenocarcinoma with mucin production. However, approximately 80% of adenocarcinomas are found to contain two (or more) of these four subtypes. Multiphasic tumors such as these are classified into a fifth "subtype", termed adenocarcinoma with mixed subtypes.[2]
There are other classification systems that have been proposed for lung cancers, including BACs and other forms of adenocarcinoma. The Noguchi classification system for small adenocarcinomas has received considerable attention, particularly in Japan, but has not been nearly as widely applied and recognized as the WHO system.[6]
Like other forms of lung carcinoma, BAC possesses unique clinical and pathological features, prognosis, and responses to different treatments.[7]
Diagnosis
The criteria for diagnosing BACs have changed since 1999.[8][9] Under the new definition, BAC is defined as a tumor that grows in a lepidic (that is, a scaly covering) fashion along pre-existing airway structures, without detectable invasion or destruction of the underlying tissue, blood vessels, or lymphatics.[10] Because invasion must be ruled out, BAC can be diagnosed only after complete sectioning and examination of the entire tumor, not using biopsy or cytology samples. BAC is considered a pre-invasive malignant lesion that, after further mutation and progression, eventually generates an invasive adenocarcinoma. Therefore, it is considered a form of carcinoma in situ (CIS).
Epidemiology
The criteria for diagnosing BAC have changed since 1999.[10] Under the new definition, BAC is not considered to be an invasive tumor by pathologists, but as one form of carcinoma in situ (CIS). Like other forms of CIS, BAC may progress and become overtly invasive, exhibiting malignant, often lethal, behavior. Major surgery, either a lobectomy or a pneumonectomy, is usually needed to control it, and like other forms of non-small cell lung carcinoma, recurrences are frequent. Therefore, oncologists classify it among the other malignant tumors, which are invasive tumors.[2]
Under the new, more restrictive WHO criteria for lung cancer classification, BAC is now diagnosed much less frequently than it was in the past.[2] Recent studies suggest that BAC comprises between 3% and 5% of all lung carcinomas in the U.S.[11][12]
Variants of BAC
BAC occurs in two major histopathological variants, mucinous BAC (m-BAC, 20–25% of cases)[13][14] and non mucinous BAC (nm-BAC, 75–80% of cases).[14][15] Very rarely, BAC can also occur as a "mixed mucinous and non-mucinous" (or "indeterminant") variant.[2]
Incidence
The incidence of bronchiolo-alveolar carcinoma has been reported to vary from 4–24% of all lung cancer patietnts.[12] An analysis of Surveillance epidemiology and End results registry ( SEER) by Read et al. revealed that although the incidence of BAC has increased over the past two decade it still constitutes less than 4% of NSCLC in every time interval.[12] This difference in the incidence has been attributed to complex histopathology of cancer. While pure BAC is rare, the increase in incidence as seen in various studies can be due to unclear histological classification till WHO came up with its classification in 1999 and then in 2004. Another distinguishing feature about BAC is that it afflicts men and women in equal proportions, some recent studies even suggest slightly higher incidence among women. [11][12]
Histogenesis
Nonmucinous BAC is thought to derive from a transformed cell in the distal airways and terminal respiratory units, and often shows features of Clara cell or Type II pneumocyte differentiation.[13] Mucinous BAC, in contrast, probably derives from a transformed glandular cell in distal bronchioles.[16]
Type-I cystic adenomatoid malformation (CAM) has recently been identified as a precursor lesion for the development of mucinous BAC, but these cases are rare.[17][18]
Rarely, BAC may develop a rhabdoid morphology due to the development of dense perinuclear inclusions.[19]
Treatment
The treatment of choice in any patient with BAC is complete surgical resection, typically via lobectomy or pneumonectomy, with concurrent ipsilateral lymphadenectomy.[14]
Non-mucinous BACs are highly associated with classical EGFR mutations, and thus are often responsive to targeted chemotherapy with erlotinib and gefinitib. K-ras mutations are rare in nm-BAC.[20]
Mucinous BAC, in contrast, is much more highly associated with K-ras mutations and wild-type EGFR, and are thus usually insensitive to the EGFR tyrosine kinase inhibitors.[21] In fact, there is some evidence that suggests that the administration of EGFR-pathway inhibitors to patients with K-ras mutated BACs may even be harmful.[4]
Recurrence
When BAC recurs after surgery, the recurrences are local in about three-quarters of cases, a rate higher than other forms of NSCLC, which tends to recur distantly.[14]
Prognosis and Survival
Taken as a class, long-term survival rates in BAC tend to be higher than those of other forms of NSCLC.[22][23] BAC generally carries a better prognosis than other forms of NSCLC, which can be partially attributed to localized presentation of the disease. [12] Though other factors might play a role. Prognosis of BAC depends upon the histological subtype and extent at presentation but are generally same as other NSCLC.[24]
Recent research has made it clear that nonmucinous and mucinous BACs are very different types of lung cancer.[13][25] Mucinous BAC is much more likely to present with multiple unilateral tumors and/or in a unilateral or bilateral pneumonic form than nonmucinous BAC.[13] The overall prognosis for patients with mucinous BAC is significantly worse than patients with nonmucinous BAC.[11][26]
Although data are scarce, some studies suggest that survival rates are even lower in the mixed mucinous/non-mucinous variant than in the monophasic forms.[26]
In non-mucinous BAC, neither Clara cell nor Type II pneumocyte differentiation appears to affect survival or prognosis.[13]
Additional images
Mucinous BAC
References
- ^ Roggli VL, Vollmer RT, Greenberg SD, McGavran MH, Spjut HJ, Yesner R (1985). "Lung cancer heterogeneity: a blinded and randomized study of 100 consecutive cases". Hum. Pathol. 16 (6): 569–79. doi:10.1016/S0046-8177(85)80106-4. PMID 2987102.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f Travis, William D; Brambilla, Elisabeth; Muller-Hermelink, H Konrad; Harris, Curtis C, eds. (2004). Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart (PDF). World Health Organization Classification of Tumours. Lyon: IARC Press. ISBN 92-832-2418-3. Retrieved 27 March 2010.
- ^ Rossi G, Marchioni A, Sartori1 G, Longo L, Piccinini S, Cavazza A (2007). "Histotype in non-small cell lung cancer therapy and staging: The emerging role of an old and underrated factor". Curr Resp Med Rev. 3: 69–77. doi:10.2174/157339807779941820.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ a b Vincent MD (2009). "Optimizing the management of advanced non-small-cell lung cancer: a personal view". Curr Oncol. 16 (4): 9–21. doi:10.3747/co.v16i4.465. PMC 2722061. PMID 19672420.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Travis WD, Travis LB, Devesa SS (1995). "Lung cancer". Cancer. 75 (1 Suppl): 191–202. doi:10.1002/1097-0142(19950101)75:1+<191::AID-CNCR2820751307>3.0.CO;2-Y. PMID 8000996.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Noguchi M, Morikawa A, Kawasaki M; et al. (1995). "Small adenocarcinoma of the lung. Histologic characteristics and prognosis". Cancer. 75 (12): 2844–52. PMID 7773933.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Varlotto JM, Flickinger JC, Recht A, Nikolov MC, DeCamp MM (2008). "A comparison of survival and disease-specific survival in surgically resected, lymph node-positive bronchioloalveolar carcinoma versus nonsmall cell lung cancer: implications for adjuvant therapy". Cancer. 112 (7): 1547–54. doi:10.1002/cncr.23289. PMID 18260087.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kreyberg, L.; Liebow, A.A.; Uehlinger, E.A.; World Health Organization. (1967). Histological Typing of Lung Tumours. International histological classification of tumours, no. 1 (1st ed.). Geneva, Switzerland: World Health Organization. OCLC 461784.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ World Health Organization (1981). Histological Typing of Lung Tumours. International histological classification of tumours, no. 1 (2nd ed.). Geneva, Switzerland: World Health Organization. ISBN 92-4-176101-6. OCLC 476258805.
- ^ a b Travis, W.D.; Colby, T.V.; Corrin, B.; et al.Histological Typing of Lung and Pleural Tumors. International histological classification of tumours, no. 1 (3rd ed.). Berlin: Springer. 1999. ISBN 3-540-65219-1.
- ^ a b c Zell JA, Ou SH, Ziogas A, Anton-Culver H (2005). "Epidemiology of bronchioloalveolar carcinoma: improvement in survival after release of the 1999 WHO classification of lung tumors". J. Clin. Oncol. 23 (33): 8396–405. doi:10.1200/JCO.2005.03.0312. PMID 16293870.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Read WL, Page NC, Tierney RM, Piccirillo JF, Govindan R (2004). "The epidemiology of bronchioloalveolar carcinoma over the past two decades: analysis of the SEER database". Lung Cancer. 45 (2): 137–42. doi:10.1016/j.lungcan.2004.01.019. PMID 15246183.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) Cite error: The named reference "ReadPage" was defined multiple times with different content (see the help page). - ^ a b c d e Yousem SA, Beasley MB (2007). "Bronchioloalveolar carcinoma: a review of current concepts and evolving issues". Arch. Pathol. Lab. Med. 131 (7): 1027–32. doi:10.1043/1543-2165(2007)131[1027:BCAROC]2.0.CO;2. PMID 17616987.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d Raz DJ, He B, Rosell R, Jablons DM (2006). "Current concepts in bronchioloalveolar carcinoma biology". Clin. Cancer Res. 12 (12): 3698–704. doi:10.1158/1078-0432.CCR-06-0457. PMID 16778095.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lee KS, Kim Y, Han J, Ko EJ, Park CK, Primack SL (1 November 1997). "Bronchioloalveolar carcinoma: clinical, histopathologic, and radiologic findings". Radiographics. 17 (6): 1345–57. PMID 9397450.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chilosi M, Murer B (2010). "Mixed adenocarcinomas of the lung: place in new proposals in classification, mandatory for target therapy". Arch. Pathol. Lab. Med. 134 (1): 55–65. doi:10.1043/1543-2165-134.1.55. PMID 20073606.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Scialpi M, Cappabianca S, Rotondo A; et al. (2010). "Pulmonary congenital cystic disease in adults. Spiral computed tomography findings with pathologic correlation and management". Radiol Med. 115 (4): 539–50. doi:10.1007/s11547-010-0467-6. PMID 20058095.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Abecasis F, Gomes Ferreira M, Oliveira A, Vaz Velho H (2008). "[Bronchioloalveolar carcinoma associated with congenital pulmonary airway malformation in an asymptomatic adolescent]". Rev Port Pneumol (in Portuguese). 14 (2): 285–90. PMID 18363023.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Song DE, Jang SJ, Black J, Ro JY (2007). "Mucinous bronchioloalveolar carcinoma of lung with a rhabdoid component—report of a case and review of the literature". Histopathology. 51 (3): 427–30. doi:10.1111/j.1365-2559.2007.02784.x. PMID 17727492.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Finberg KE, Sequist LV, Joshi VA; et al. (2007). "Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features". J Mol Diagn. 9 (3): 320–6. doi:10.2353/jmoldx.2007.060182. PMC 1899415. PMID 17591931.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sakuma Y, Matsukuma S, Yoshihara M; et al. (2007). "Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations". Am. J. Clin. Pathol. 128 (1): 100–8. doi:10.1309/WVXFGAFLAUX48DU6. PMID 17580276.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Breathnach OS, Kwiatkowski DJ, Finkelstein DM; et al. (2001). "Bronchioloalveolar carcinoma of the lung: recurrences and survival in patients with stage I disease". J. Thorac. Cardiovasc. Surg. 121 (1): 42–7. doi:10.1067/mtc.2001.110190. PMID 11135158.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Grover FL, Piantadosi S (1989). "Recurrence and survival following resection of bronchioloalveolar carcinoma of the lung--The Lung Cancer Study Group experience". Ann. Surg. 209 (6): 779–90. PMC 1494125. PMID 2543339.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Barkley, JE (1996 Aug). "Bronchioloalveolar carcinoma". Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 14 (8): 2377–86. PMID 8708731.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Garfield DH, Cadranel J, West HL (2008). "Bronchioloalveolar carcinoma: the case for two diseases". Clin Lung Cancer. 9 (1): 24–9. doi:10.3816/CLC.2008.n.004. PMID 18282354.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Furák J, Troján I, Szoke T; et al. (2003). "Bronchioloalveolar lung cancer: occurrence, surgical treatment and survival". Eur J Cardiothorac Surg. 23 (5): 818–23. PMID 12754039.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)