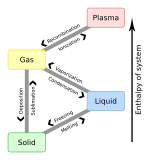
Sublimation (phase transition)


At normal pressures, most chemical compounds and elements possess three different states at different temperatures. In these cases, the transition from the solid to the gaseous state requires an intermediate liquid state. Note, however, that the pressure referred to here is the partial pressure of the substance, not the total (e.g., atmospheric) pressure of the entire system. So, all solids that possess an appreciable vapor pressure at a certain temperature usually can sublime in air (e.g., water ice just below 0°C). For some substances, such as carbon and arsenical, sublimation is much easier than evaporation from the melt, because the pressure of their triple point is very high, and it is difficult to obtain them as liquids.
Sublimation requires additional energy and is an endothermic change. The enthalpy of sublimation (also called heat of sublimation) can be calculated as the enthalpy of fusion plus the enthalpy of vaporization. The reverse process of sublimation is deposition. The formation of frost is an example of meteorological deposition.
Examples
Carbon dioxide

Solid carbon dioxide (dry ice) sublimes readily at atmospheric pressure at -78.5°C (197.5 K, −104.2 °F), whereas liquid CO2 can be obtained at pressures and temperatures above the triple point (5.2 atm, -56.4°C).
Water
Snow and ice sublime, although more slowly, below the melting point temperature.[1] This allows a wet cloth to be hung outdoors in freezing weather and retrieved later in a dry state. In freeze-drying, the material to be dehydrated is frozen and its water is allowed to sublime under reduced pressure or vacuum. The loss of snow from a snowfield during a cold spell is often caused by sunshine acting directly on the upper layers of the snow. Ablation is a process that includes sublimation and erosive wear of ice.
Other compounds

Iodine produces fumes on gentle heating. It is possible to obtain liquid iodine at atmospheric pressure by controlling the temperature at just above the melting point of iodine. In forensic science, iodine vapor can reveal latent fingerprints on paper.[2]
Naphthalene, a common ingredient in mothballs, also sublimes easily. Arsenic can also sublime at high temperatures.
Various substances appear to sublime because of undergoing chemical reactions or decomposition; for example, ammonium chloride when heated decomposes into hydrogen chloride and ammonia.
Sublimation purification

Sublimation is a technique used by chemists to purify compounds. A solid is typically placed in a sublimation apparatus and heated under vacuum. Under this reduced pressure, the solid volatilizes and condenses as a purified compound on a cooled surface (cold finger), leaving a non-volatile residue of impurities behind. Once heating ceases and the vacuum is removed, the purified compound may be collected from the cooling surface.[3][4]
Historical usage
In alchemy, sublimation can refer to the process in which a substance is heated to a vapor, then immediately collects as sediment on the upper portion and neck of the heating medium (typically a retort or alembic), but can also be used to describe other similar non-laboratory transitions. It is mentioned by alchemical authors such as Basil Valentine and George Ripley, and in the Rosarium philosophorum, as a process necessary for the completion of the magnum opus. Here, the word sublimation is used to describe an exchange of "bodies" and "spirits" similar to laboratory phase transition between solids and gases. Valentine, in his Triumphal Chariot of Antimony makes a comparison to spagyrics in which a vegetable sublimation can be used to separate the spirits in wine and beer.[5] Ripley uses language more indicative of the mystical implications of sublimation, indicating that the process has a double aspect in the spiritualization of the body and the corporalizing of the spirit.[6] He writes:
- And Sublimations we make for three causes,
The first cause is to make the mind spiritual.
The second is that the spirit may be corporeal,
And become fixed with it and consubstantial.
The third cause is that from its filthy original.
It may be cleansed, and its saltiness sulphurious
May be diminished in it, which is infectious.[7]
- And Sublimations we make for three causes,
See also
- Ablation
- Phase diagram
- Cold trap
- Enthalpy of sublimation
- Dye-sublimation printer, Freezer burn - common processes involving sublimation
To From
|
Solid | Liquid | Gas | Plasma |
|---|---|---|---|---|
| Solid | Melting | Sublimation | ||
| Liquid | Freezing | Vaporization | ||
| Gas | Deposition | Condensation | Ionization | |
| Plasma | Recombination |
References
- ^ S. R. Fassnacht, Estimating Alter-shielded gauge snowfall undercatch, snowpack sublimation, and blowing snow transport at six sites in the coterminous USA, Hydrol. Process. 18, 3481–3492 (2004)
- ^ James Girard (2011). Criminalistics: Forensic Science, Crime and Terrorism. Jones & Bartlett Learning. pp. 143–144. ISBN 0-7637-7731-5, ISBN 978-0-7637-7731-9.
{{cite book}}: Check|isbn=value: invalid character (help) - ^ King, R. B. Organometallic Syntheses. Volume 1 Transition-Metal Compounds; Academic Press: New York, 1965. ISBN 0-444-42607-8.
- ^ Laurence M. Harwood, Christopher J. Moody (13 Jun 1989). Experimental organic chemistry: Principles and Practice (Illustrated edition ed.). WileyBlackwell. pp. 154–155. ISBN 0-632-02017-2, ISBN 978-0-632-02017-1.
{{cite book}}:|edition=has extra text (help); Check|isbn=value: invalid character (help) - ^ Francis Barrett. The lives of alchemystical philosophers: with a critical catalogue of books in occult chemistry, and a selection of the most celebrated treatises on the theory and practice of the hermetic art. Printed by Macdonald and son, for Lackington, Allen, & co., 1815. p.233
- ^ Barbara DiBernard. Alchemy and Finnegans wake. SUNY Press, 1980. p.57
- ^ George Ripley. Compoundof Alchemy. 1591. Retrieved from http://www.levity.com/alchemy/ripgat8.html