Vertebral augmentation
| Percutaneous vertebroplasty | |
|---|---|
 Typical interventional suite setup for vertebroplasty | |
| ICD-9-CM | 81.65 |
| MedlinePlus | 007512 |
Vertebroplasty and kyphoplasty are similar medical spinal procedures where acrylic bone cement is injected through a small hole in the skin (percutaneously) into a fractured vertebra with the goal of relieving the pain of vertebral compression fractures (VCF). In 2009 Vertebroplasty was found to be ineffective in treating compression fracture of the spine in two randomized placebo controlled trials done to study the procedure that used a sham procedure for the control group.[1]. These initial findings were discordant with the earlier positive findings. Since then there has been new clinical literature in support of both vetebralplasty and balloon kyphoplasty.
Effectiveness
These procedures have some amount of uncertainty with regard to the overall benefit, with some newer evidence finding benefit while others find limited benefit. Two randomized and blinded trials found limited benefit however they have been faulted for not looking at people with acute vertebral fractures.[2] Some have suggested that this procedure only be done in those with fractures less than 6 weeks old (which was not the population of these two trials).[3] Other consider the procedure only in those with other health problems where rest could be detrimental, those with metastatic cancer as the cause of the spine fracture, or those who do not improve with conservative management.[4]
NEJM Articles
Two studies published in The New England Journal of Medicine in 2009 found no benefit to vertebroplasty for compression fractures when compared to a sham procedure :[5]
- In a multicenter, prospective double-blinded randomized controlled trial (RCT) involving 131 participants who were patients with one or two painful osteoporotic vertebral fractures, vertebroplasty did not result in greater improvement than a sham procedure in overall pain, physical functioning, or quality of life at 3 or 6 months after treatment.[6] Jeffrey Jarvik of the University of Washington said his study, funded by the National Institutes of Health, found vertebroplasty had no detectable benefit when compared with procedures that only mimicked such procedures. He advises that "vertebroplasty should not be done any longer, unless it's in the setting of a study."[citation needed]
- In a multicenter, randomized, double-blind, placebo-controlled trial involving 78 participants with osteoporotic vertebral compression fractures, people who underwent vertebroplasty had improvements in pain and disability measures that were similar to those in patients who underwent a sham procedure.[7] University of Virginia radiologist Avery Evans said his study, which was funded by the Australian government and Cook Medical Inc., found vertebroplasty and sham procedures offered patients nearly identical pain relief.[5]
Several case reports and unblinded studies initially suggested that vertebroplasty provided effective relief of pain.[8][9][10][11] However, none of these studies were comparisons to a placebo.
Nevertheless, many vertebroplasty practitioners and healthcare professional organizations continue to advocate for the procedure.[12][13]
VERTOS II Study
An unblinded study published in 2010 compared vertebroplasty to conservative care with 202 patients. The results were consistent with prior unblinded studies; patients who, knowingly, underwent vertebroplasty reported improvement in pain.[14]
Procedure
Vertebroplasty is typically performed by a spine surgeon or interventional radiologist. It is a minimally invasive procedure and patients usually go home the same or next day as the procedure. Patients are given local anesthesia and light sedation for the procedure, though it can be performed using only local anesthetic for patients with medical problems who cannot tolerate sedatives well.
During the procedure, bone cement is injected with a biopsy needle into the collapsed or fractured vertebra. The needle is placed with fluoroscopic x-ray guidance. The cement (most commonly PMMA, although more modern cements are used as well) quickly hardens and forms a support structure within the vertebra that provide stabilization and strength. The needle makes a small puncture in the patient's skin that is easily covered with a small bandage after the procedure.[15]
Kyphoplasty
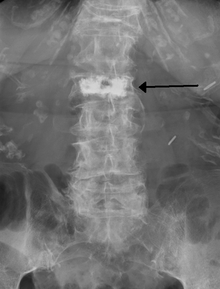
Kyphoplasty is a variation of a vertebroplasty that attempts to stop the pain caused by the bone fracture and attempts to restore the height and angle of kyphosis of a fractured vertebra (of certain types), followed by its stabilization using injected bone cement. The procedure typically includes the use of a small balloon that is inflated in the vertebral body to create a void within the cancellous bone prior to cement delivery.[16] Once the void is created, the procedure continues in a similar manner as a vertebroplasty, but the bone cement is typically delivered directly into the newly created void.
In its review of vertebroplasty and vertebral augmentation procedures, Medicare contractor NAS determined that there is no difference between vertebroplasty and kyphoplasty, stating, "No clear evidence demonstrates that one procedure is different from another in terms of short- or long-term efficacy, complications, mortality or any other parameter useful for differentiating coverage."[17]
Effectiveness
Several unblinded clinical studies have suggested a benefit of balloon kyphoplasty for patients with spinal fractures. Earlier unblinded studies also suggested a similar benefit to the closely related procedure vertebroplasty, however the only two blinded randomized controlled studies done to assess vertebroplasty failed to demonstrate any benefit as compared to patients who received a sham procedure.[18][19] Although no blinded studies have been performed on kyphoplasty, since the procedure is a derivative of vertebroplasty, the unsuccessful results of these blinded studies have cast doubt upon the benefit of kyphoplasty despite the continued benefit suggested by unblinded studies.[20]
Adverse effects

Some of the associated risks that can be produced are from the leak of acrylic cement outside of the vertebral body. Although severe complications are extremely rare, infection, bleeding, numbness, tingling, headache, and paralysis may ensue due to misplacement of the needle or cement. This particular risk is decreased by the use of x-ray or other radiological imaging to ensure proper placement of the cement.[15]
The risk of new fractures following these procedures does not appear to be changed, however evidence is limited,[21] and an increase risk as of 2012 is not ruled out.[22]
Pulmonary cement embolism is reported to occur in approximately 2-26% of procedures.[23] It may occur with or without symptoms.[23] If there are no symptoms there is typically no long term issues.[23]
History
Vertebroplasty had been performed as an open procedure for many decades to secure pedicle screws and fill tumorous voids. However, the results were not always worth the risk involved with an open procedure, which was the reason for the development of percutaneousvertebroplasty.
The first percutaneous vertebroplasty was performed in 1984 at the University Hospital of Amiens, France to fill a vertebral void left after the removal of a benign spinal tumor. A report of this and 6 other patients was published in 1987 and it was introduced in the United States in the early 1990s. Initially, the treatment was used primarily for tumors in Europe and VCF in the United States, although the distinction has largely gone away since then.[24]
Society and culture
Medicare response to NEJM articles
In response to the NEJM articles and a medical record review showing misuse of vertebroplasty and kyphoplasty, US Medicare contractor Noridian Administrative Services (NAS) conducted a literature review and formed a policy regarding reimbursement of the procedures. NAS states that in order to be reimbursable, a procedure must meet a certain criteria, including, 1) a detailed and extensively documented medical record showing pain caused by a fracture, 2) radiographic confirmation of a fracture, 3) that other treatment plans were attempted for a reasonable amount of time, 4) that the procedure is not performed in the emergency department, and 5) that at least 1 year of follow-up is planned for, among others. The policy, as referenced, applies only to the region covered by Noridian and not all of Medicare's coverage area. It became effective on 20 June 2011 and remains current.[17]
AAOS recommendation
On September 4th, 2010, the Board of Directors of the American Academy of Orthopaedic Surgeons (AAOS) released The Treatment of Symptomatic Osteoporotic Spinal Compression Fractures: Guideline and Evidence Report containing a "Strong" recommendation against use of vertebroplasty for osteoporotic spinal compression fractures.[25]
See also
References
- ^ Robinson, Y (2012 May). "Vertebroplasty and kyphoplasty--a systematic review of cement augmentation techniques for osteoporotic vertebral compression fractures compared to standard medical therapy". Maturitas. 72 (1): 42–9. PMID 22425141.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gangi, A (2010 Aug). "Have recent vertebroplasty trials changed the indications for vertebroplasty?". Cardiovascular and interventional radiology. 33 (4): 677–80. doi:10.1007/s00270-010-9901-3. PMID 20523998.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Clark, WA (2010-03-15). "Vertebroplasty for painful acute osteoporotic vertebral fractures: recent Medical Journal of Australia editorial is not relevant to the patient group that we treat with vertebroplasty". The Medical journal of Australia. 192 (6): 334–7. PMID 20230351.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Itshayek, E (2012 Jun). "Vertebral augmentation in the treatment of vertebral compression fractures: review and new insights from recent studies". Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 19 (6): 786–91. PMID 22595547.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b "Studies question impact of vertebroplasty." Aug. 6, 2009: UPI.com
- ^ Kallmes, DF (2009-08-06). "A randomized trial of vertebroplasty for osteoporotic spinal fractures". The New England Journal of Medicine. 361 (6): 569–79. doi:10.1056/NEJMoa0900563. PMC 2930487. PMID 19657122.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Buchbinder, R (2009-08-06). "A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures". The New England Journal of Medicine. 361 (6): 557–68. doi:10.1056/NEJMoa0900429. PMID 19657121.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ [1]
- ^ Hulme PA, Krebs J, Ferguson SJ, Berlemann U (2006). "Vertebroplasty and Kyphoplasty: A Systematic Review of 69 Clinical Studies". Spine. 31 (17): 1983–2001. doi:10.1097/01.brs.0000229254.89952.6b. PMID 16924218.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ McGraw JK, Lippert JA, Minkus KD, Rami PM, Davis TM, Budzik RF. "Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and follow-up." Journal of Vascular and Interventional Radiology 2002;13(9 pt 1):883-886.
- ^ Layton, KF et al. "Vertebroplasty, First 1000 Levels of a Single Center: Evaluation of the Outcomes and Complications." American Journal of Neuroradiology April 2007,28:683-89
- ^ Moan R. Debate continues over value of vertebroplasty. Diagnostic Imaging. 2010;32(2):5.
- ^ Jensen, ME (2009 Jul). "Position statement on percutaneous vertebral augmentation: a consensus statement developed by the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, American Association of Neurological Surgeons/Congress of Neurological Surgeons, and American Society of Spine Radiology". Journal of vascular and interventional radiology : JVIR. 20 (7 Suppl): S326-31. doi:10.1016/j.jvir.2009.04.022. PMID 19560019.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Klazen CA, Lohle PN, de Vries J; et al. (2010). "Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomized trial". Lancet. 376 (9746): 1085–92. doi:10.1016/S0140-6736(10)60954-3. PMID 20701962.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Nicole Berardoni M.D, Paul Lynch M.D, and Tory McJunkin M.D. "Vertebroplasty and Kyphoplasty" 2008. Accessed 7 Aug 2009. http://www.arizonapain.com/Vertebroplasty-W.html
- ^ Ty Thaiyananthan M.D and Bryan Oh M.D. "Kyphoplasty/Vertebroplasty Procedure Ilustrations" 2011. http://www.basicspine.com/conditions-procedures/spine-treatments/kyphoplasty.html
- ^ a b Noridian Administrative Services, LLC. "Local Coverage Determination (LCD) for Vertebroplasty, Vertebral Augmentation; Percutaneous (L24383)". Centers for Medicare and Medicaid Services. United States Department of Health and Human Services. Retrieved 18 October 2011.
- ^ Kallmes DF, Comstock BA, Heagerty PJ; et al. (2009). "A randomized trial of vertebroplasty for osteoporotic spinal fractures". N. Engl. J. Med. 361 (6): 569–79. doi:10.1056/NEJMoa0900563. PMC 2930487. PMID 19657122.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Buchbinder R, Osborne RH, Ebeling PR; et al. (2009). "A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures". N. Engl. J. Med. 361 (6): 557–68. doi:10.1056/NEJMoa0900429. PMID 19657121.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ [2]
- ^ Zou, J (2012 Jul-Aug). "The long-term incidence of subsequent vertebral body fracture after vertebral augmentation therapy: a systemic review and meta-analysis". Pain physician. 15 (4): E515-22. PMID 22828697.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Bliemel, C (2012 Apr). "Higher incidence of new vertebral fractures following percutaneous vertebroplasty and kyphoplasty--fact or fiction?". Acta orthopaedica Belgica. 78 (2): 220–9. PMID 22696994.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Wang, LJ (2012 Aug). "Pulmonary cement embolism associated with percutaneous vertebroplasty or kyphoplasty: a systematic review". Orthopaedic surgery. 4 (3): 182–9. PMID 22927153.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Mathis, John M.; Deramond, Hervé; Belkoff, Stephen M., eds. (2006) [First edition published 2002]. Percutaneous Vertebroplasty and Kyphoplasty (2nd ed.). Springer Science+Business Media. pp. 3–5. ISBN 0-387-29078-8.
- ^ Esses, Stephen I.; et al. (2010), The Treatment of Symptomatic Osteoporotic Spinal Compression Fractures: Guideline and Evidence Report (PDF), American Academy of Orthopaedic Surgeons
{{citation}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)
