Endovascular aneurysm repair
| Endovascular aneurysm repair | |
|---|---|
 Endovascular aneurysm repair | |
| ICD-9-CM | 39.51, 39.52, 39.7 |
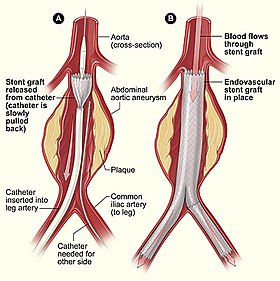
Endovascular aneurysm repair (or endovascular aortic repair) (EVAR) is a type of endovascular surgery used to treat an abdominal aortic aneurysm (AAA) or thoracic aortic aneurysm, the procedure then specifically termed TEVAR (thoracic endovascular aortic/aneurysm repair). In certain occasions, a specially designed custom-made graft device ("endograft", which has holes (fenestrations) on the graft body to maintain the patency of certain important blood vessels, is used for the procedure, which is called FEVAR (fenestrated endovascular aortic/aneurysm repair). Standard EVAR is appropriate for aneurysms that begin below the renal arteries, where there exists an adequate length of normal aorta (the "proximal aortic neck") for reliable attachment of the endograft without leakage of blood around the device ("endoleak").
History
The world's first EVAR was performed in 1987 by Nicholas Volodos in Kharkov, Soviet Union and introduced in an article written in 1988.[1] In Argentina it was first introduced in 1991 by Juan Parodi[2] and the very same year in the USA by Michael Dake.[3]
Patient screening
Before patients are deemed to be a suitable candidate for this treatment, they have to go through a rigorous set of tests. These include a CT scan of the complete thorax/abdomen/pelvis, and blood tests. The CT scan gives precise measurements of the aneurysm and the surrounding anatomy. In particular the calibre/tortuosity of the iliac arteries and the relationship of the neck of the aneurysm to the renal arteries are important determinants of whether the aneurysm is amenable to endoluminal repair. In certain occasions that the renal arteries are too close to the aneurysm, the custom-made fenestrated graft stent is now an accepted alternative to doing open surgery.
The procedure

The procedure is carried out in a sterile environment under x-ray fluoroscopic guidance. It is usually carried out by an interventional radiologist or vascular surgeon. The patient is either given a full GA (general anaestheic) or regional anaesthesia.
Vascular 'sheaths' are introduced into the patient's femoral arteries, through which the guidewires, catheters and eventually, the Stent Graft is passed.
Diagnostic angiography images or 'runs' are captured of the aorta to determine the location on the patient's renal arteries, so the stent graft can be deployed without blocking these. Failure to achieve this will cause renal failure, thus the precision and control of the graft stent deployment is extremely important. The main 'body' of the stent graft is placed first, follow by the 'limbs' which join on to the main body and sit on the Aortic Bifurcation for better support, and extend to the Iliac arteries.
In about 25-30% of the occasions, the Abdominal aneurysm extends down to the Common Iliac Arteries, a specially designed graft stent, named as Iliac Branch Device (IBD), can be used, instead of blocking the Internal Iliac Arteries, but to preserve them. The preservation of the Internal Iliac Arteries is important to prevent Buttock Claudication, and to preserve the maximum genital function.
The idea is that the stent graft (covered stent), once in place acts as an artificial lumen for blood to flow down, and not into the surrounding aneurysm sac. This therefore immediately takes the pressure off the aneurysm wall, which itself will thrombose in time.[4]

A newer adaption of EVAR is the Hybrid Procedure. A hybrid procedure occurs in the angiography room and aims to combine endovascular procedures with limited open surgery. In this procedure the stent graft deployment is planned to combine with an open operation to revascularise selected arteries that will be "covered" by the stent graft i.e. deprived of arterial inflow. In this method more extensive EVAR devices can be deployed to treat the primary lesion while preserving arterial flow to critical arteries.
Thoraco-abdominal aortic aneurysms (TAAA) typically involves such vessels and deployment of the EVAR device will cover important arteries e.g. visceral or renal arteries, resulting in end organ ischaemia which may not be survivable. The open operation component aims to bring a bypass graft from an artery outside the stent graft coverage to vital arteries within the coverage region. This component adds to the EVAR procedure in time and risk but is usually judged to be lesser that the risk of the major totally open operation.
Staging such procedures is common. A common example is revascularisation of the left common carotid artery and/or the left subclavian artery from the innominate artery or the right common carotid artery to allow treatment of a thoracic aortic aneurysm that encroachs proximally into the aortic arch to be treated without thoracotomy. Continued design improvement in stent graft including branched endografts will reduce but not eliminate this category of surgery. 'Chimney stents into the innominate artery with TEVAR into the proximal aortic arch have recently been described. Other surgeons favor limited thoracotomy with carotid-carotid bypass i.e. hybrid procedures.
All such procedures aim to reduce the morbidity and mortality of treating arterial disease in a patient population that is increasingly older and less fit than when major open repairs were developed and popularised. Even in those days, significant risks were accepted in the understanding than the large open operation was the only option. That is not the case in most patients today. Durability and problems such as 'endoleaks' may require careful surveillance and adjuvant procedures to ensure success of the EVAR or EVAR/hybrid procedure. CT Angiography (CTA) imaging has in particular made a key contribution to planning, success, durabity in this complex area of vascular surgery.
As for the latest research result, EVAR has almost exactly same mortality and morbidity, as in comparison of Open Surgery, 2 years after the procedure. However, EVAR has a significant better mortality and morbidity within 2-years post-Op. Thus in general, patients with AAA/TAA are receiving benefits from such procedure.
Complications
Systemic
Myocardial infarction, congestive heart failure, arrhythmias, respiratory failure, renal failure
Dissection, malpositioning, renal failure, thromboembolizaton, ischemic colitis, groin hematoma, wound infection
Migration, detachment, rupture, stenosis, kinking
Endoleaks
An endoleak is a leak into the aneurysm sac after endovascular repair. Five types of endoleaks exist:[4]
- Type I - Perigraft leakage at proximal or distal graft attachment sites (near the renal and iliac arteries)
- Type II - Retrograde flow from collateral branches such as the lumbar, testicular and inferior mesenteric arteries
- Type III - Leakage between overlapping parts of the stent (i.e. anastomosis between overlapping components) or rupture through graft material.
- Type IV - Leakage through the graft wall due to the quality (porosity) of the graft material
- Type V - Leakage from unknown origin
Use in aortic dissection
In uncomplicated type B aortic dissection, TEVAR does not seem either improve or compromise 2-year survival and adverse event rates.[5] Its use in complicated aortic dissection is under investigation.
References
- ^ Volodos' NL, Karpovich IP, Shekhanin VE, et al. A case of distant transfemoral endoprosthesis of the thoracic artery using a self-fixing synthetic prosthesis in traumatic aneurysm]. [Article in Russian] Grudn Khir. 1988 Nov-Dec;(6):84-6. PMID 3220297 [PubMed - indexed for MEDLINE]
- ^ Aho PS (2006). Expectations pf Endovascular Repair of Abdominal Aortic Aneurysm Academic dissertation.
- ^ Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. Dec 29 1994;331(26):1729-34. [Medline]
- ^ a b Greenhalgh RM, Powell JT (2008). "Endovascular repair of abdominal aortic aneurysm". N. Engl. J. Med. 358 (5): 494–501. doi:10.1056/NEJMct0707524. PMID 18234753.
- ^ Nienaber CA, Rousseau H, Eggebrecht H; et al. (2009). "Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial". Circulation. 120 (25): 2519–28. doi:10.1161/CIRCULATIONAHA.109.886408. PMID 19996018.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
