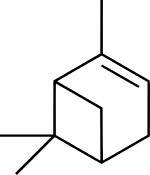
Pinene
| |
| Names | |
|---|---|
| IUPAC name
(1S,5S)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene
(1S,5S)-6,6-dimethyl-2-methylenebicyclo[3.1.1]heptane
| |
| Identifiers | |
| ECHA InfoCard | 100.029.170 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C10H16 | |
| Molar mass | 136.24 g/mol |
| Appearance | Liquid |
| Density | 0,86 g·cm−3 (alpha, 15 °C)[1][2] |
| Melting point | −62 to −55 °C (−80 to −67 °F; 211 to 218 K) |
| Boiling point | 155 to 156 °C (311 to 313 °F; 428 to 429 K) |
| Practically insoluble in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
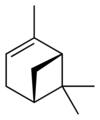
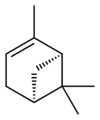
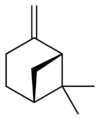
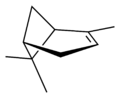
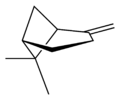
Pinene (C10H16) is a bicyclic monoterpene chemical compound.[1] There are two structural isomers of pinene found in nature: α-pinene and β-pinene. As the name suggests, both forms are important constituents of pine resin; they are also found in the resins of many other conifers, as well as in non-coniferous plants such as big sagebrush (Artemisia tridentata). Both isomers are used by many insects in their chemical communication system. The two isomers of pinene consititute the major component of turpentine.
Isomers
 |
 |
 |
 | |
 |
 | |||
 |
 | |||
Biosynthesis
α-Pinene and β-pinene are both produced from geranyl pyrophosphate, via cyclisation of linaloyl pyrophosphate followed by loss of a proton from the carbocation equivalent.

Plants
Alpha-pinene is the most widely encountered terpenoid in nature[3] and is highly repellant to insects.[4]
Alpha-pinene appears in conifers and numerous other plants.[5] Pinene is a major component of the essential oils of Sideritis spp. (ironwort)[6] and Salvia spp. (sage).[7] Cannabis also contains alpha-pinene.[5] Resin from Pistacia terebinthus (commonly known as terebinth or turpentine tree) is rich in pinene. Pine nuts produced by pine trees contain pinene.[5]
Makrut lime fruit peel contains an essential oil comparable to lime fruit peel oil; its main components are limonene and β-pinene.[8]
Usage
In chemical industry, selective oxidation of pinene with some catalysts gives many compounds for perfumery, such as artificial odorants. An important oxidation product is verbenone, along with pinene oxide, verbenol and verbenyl hydroperoxide. [9]

Pinenes are the primary constituents of turpentine.
References
- ^ a b c Record of alpha-Pinen in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 24. January 2008.
- ^ Record of beta-Pinen in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 24. January 2008.
- ^ Noma Y, Asakawa Y (2010). Biotransformation of monoterpenoids by microorganisms, insects, and mammals. In: Baser KHC, Buchbauer G (eds). Handbook of Essential Oils: Science, Technology, and Applications. CRC Press: Boca Raton, FL, pp. 585–736.
- ^ "Repellent activity of essential oils: a review". Bioresour Technol. 101 (1): 372–378. 2010. doi:10.1016/j.biortech.2009.07.048. PMID 19729299.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ a b c Russo, E. B (2011). "Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects". British Journal of Pharmacology. 163 (7): 1344–1364. doi:10.1111/j.1476-5381.2011.01238.x. PMC 3165946. PMID 21749363.
- ^ Kose EO, Deniz IG, Sarikurkcu C, Aktas O, Yavuz M (2010). Chemical composition, antimicrobial and antioxidant activities of the essential oils of Sideritis erythrantha Boiss. and Heldr. (var. erythrantha and var. cedretorum P.H. Davis) endemic in Turkey. Food Chem Toxicol 48: 2960–2965.
- ^ Ozek G, Demirci F, Ozek T, Tabanca N, Wedge DE, Khan SI et al. (2010). Gas chromatographic-mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm., and evaluation for biological activity. J Chromatog 1217: 741–748.
- ^ Kasuan, Nurhani (2013). "Extraction of Citrus hystrix D.C. (Kaffir Lime) Essential Oil Using Automated Steam Distillation Process: Analysis of Volatile Compounds" (PDF). Malyasian Journal of Analytical Sciences. 17 (3): 359–369.
- ^ U. Neuenschwander (2010), "Mechanism of the Aerobic Oxidation of α-Pinene", ChemSusChem, vol. 3, no. 1, pp. 75–84, doi:10.1002/cssc.200900228
Bibliography
- Mann, J.; Davidson, R. S.; Hobbs, J. B.; Banthorpe, D. V.; Harborne, J. B. (1994). Natural Products. Harlow, UK: Addison Wesley Longman Ltd. pp. 309–311. ISBN 0-582-06009-5.
