Organochlorine chemistry
 
|
| Two representations of the organochloride chloroform. |
An organochloride', organochlorine compound', chlorocarbon, chlorinated hydrocarbon, is an organic compound containing at least one covalently bonded atom of chlorine as the dominant functionality, of which chloroalkanes and chlorinated solvents are major members. Their wide structural variety and divergent chemical properties lead to a broad range of names and applications. Many such compounds are controversial because of the effects of these compounds on the environment and on human and animal health.[citation needed]
Physical and chemical properties
Chlorination modifies the physical properties of hydrocarbons in several ways. The compounds are typically denser than water due to the higher atomic weight of chlorine vs hydrogen. Aliphatic organochlorides are alkylating agents because chloride is a leaving group.
Natural occurrence
Many organochlorine compounds have been isolated from natural sources ranging from bacteria to humans.[1][2] Chlorinated organic compounds are found in nearly every class of biomolecules including alkaloids, terpenes, amino acids, flavonoids, steroids, and fatty acids.[1][3] Organochlorides, including dioxins, are produced in the high temperature environment of forest fires, and dioxins have been found in the preserved ashes of lightning-ignited fires that predate synthetic dioxins.[4] In addition, a variety of simple chlorinated hydrocarbons including dichloromethane, chloroform, and carbon tetrachloride have been isolated from marine algae.[5] A majority of the chloromethane in the environment is produced naturally by biological decomposition, forest fires, and volcanoes.[6] The natural organochloride epibatidine, an alkaloid isolated from tree frogs, has potent analgesic effects and has stimulated research into new pain medication.
Preparation
From chlorine
Alkanes and arylalkanes may be chlorinated under free radical conditions, with UV light. However, the extent of chlorination is difficult to control. Aryl chlorides may be prepared by the Friedel-Crafts halogenation, using chlorine and a Lewis acid catalyst.
The haloform reaction, using chlorine and sodium hydroxide, is also able to generate alkyl halides from methyl ketones, and related compounds. Chloroform was formerly produced thus.
Chlorine adds to the multiple bonds on alkenes and alkynes as well, giving di- or tetra-chloro compounds.
Reaction with hydrogen chloride
Alkenes react with hydrogen chloride (HCl) to give alkyl chlorides. For example, the industrial production of chloroethane proceeds by the reaction of ethylene with HCl:
- H2C=CH2 + HCl → CH3CH2Cl
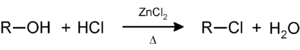
Secondary and tertiary alcohols react with the Lucas reagent (zinc chloride in concentrated hydrochloric acid) to give the corresponding alkyl halide; this reaction a method for classifying alcohols:
Other chlorinating agents
In the laboratory, alkyl chlorides are most easily prepared by reacting alcohols with thionyl chloride (SOCl2), phosphorus trichloride (PCl3), or phosphorus pentachloride (PCl5):
- ROH + SOCl2 → RCl + SO2 + HCl
- 3 ROH + PCl3 → 3 RCl + H3PO3
- ROH + PCl5 → RCl + POCl3 + HCl
In the laboratory, thionyl chloride is especially convenient, because the byproducts are gaseous.
Alternatively, the Appel reaction:
Reactions
Alkyl chlorides are versatile building blocks in organic chemistry. While alkyl bromides and iodides are more reactive, alkyl chlorides tend to be less expensive and more readily available. Alkyl chlorides readily undergo attack by nucleophiles.
Heating alkyl halides with sodium hydroxide or water gives alcohols. Reaction with alkoxides or aroxides give ethers in the Williamson ether synthesis; reaction with thiols give thioethers. Alkyl chlorides readily react with amines to give substituted amines. Alkyl chlorides are substituted by softer halides such as the iodide in the Finkelstein reaction. Reaction with other pseudohalides such as azide, cyanide, and thiocyanate are possible as well. In the presence of a strong base, alkyl chlorides undergo dehydrohalogenation to give alkenes or alkynes.
Alkyl chlorides react with magnesium to give Grignard reagents, transforming an electrophilic compound into a nucleophilic compound. The Wurtz reaction reductively couples two alkyl halides to couple with sodium.
Applications
Vinyl chloride
The largest application of organochlorine chemistry is the production of vinyl chloride. The annual production in 1985 was around 13 billion kilograms, almost all of which was converted into polyvinylchloride (PVC).
Chloromethanes
Most low molecular weight chlorinated hydrocarbons such as chloroform, dichloromethane, dichloroethene, and trichloroethane are useful solvents. These solvents tend to be relatively non-polar; they are therefore immiscible with water and effective in cleaning applications such as degreasing and dry cleaning. Several billion kilograms of chlorinated methanes are produced annually, mainly by chlorination of methane:
- CH4 + x Cl2 → CH4−xClx + x HCl
The most important is dichloromethane, which is mainly used as a solvent. Chloromethane is a precursor to chlorosilanes and silicones. Historically significant, but smaller in scale is chloroform, mainly a precursor to chlorodifluoromethane (CHClF2) and tetrafluoroethene which is used in the manufacture of Teflon.[7]
Pesticides
The two main groups of organochlorine insecticides are the DDT-type compounds and the chlorinated alicyclics. Their mechanism of action differs slightly: The DDT like compounds work on the peripheral nervous system. At the axon's sodium channel, they prevent gate closure after activation and membrane depolarization. Sodium ions ions leak through the nerve membrane and create a destabilizing negative "afterpotential" with hyperexcitability of the nerve. This leakage causes repeated discharges in the neuron either spontaneously or after a single stimulus.[8]: 255
Chlorinated cyclodienes include aldrin, dieldrin, endrin, heptachlor, chlordane and endosulfan. A 2- to 8-hour exposure leads to depressed central nervous system (CNS) activity, followed by hyperexcitability, tremors, and then seizures. The mechanism of action is the insecticide binding at the GABAA site in the gamma-Aminobutyric acid (GABA) chloride ionophore complex, which inhibits chloride flow into the nerve.[8]: 257
Other examples include dicofol, mirex, kepone and pentachlorophenol. These can be either hydrophilic or hydrophobic depending on their molecular structure.[9]
Insulators
Polychlorinated biphenyls (PCBs) were once commonly used electrical insulators and heat transfer agents. Their use has generally been phased out due to health concerns. PCBs were replaced by polybrominated diphenyl ethers (PBDEs), which bring similar toxicity and bioaccumulation concerns.
Toxicity
Some types of organochlorides have significant toxicity to plants or animals, including humans. Dioxins, produced when organic matter is burned in the presence of chlorine, and some insecticides, such as DDT, are persistent organic pollutants which pose dangers when they are released into the environment. For example, DDT, which was widely used to control insects in the mid 20th century, also accumulates in food chains, and causes reproductive problems (e.g., eggshell thinning) in certain bird species.[10]
However, the presence of chlorine in an organic compound does not ensure toxicity. Some organochlorides are considered safe enough for consumption in foods and medicines. For example, peas and broad beans contain the natural chlorinated plant hormone 4-chloroindole-3-acetic acid (4-Cl-IAA);[11][12] and the sweetener sucralose (Splenda) is widely used in diet products. As of 2004[update], at least 165 organochlorides had been approved worldwide for use as pharmaceutical drugs, including the natural antibiotic vancomycin, the antihistamine loratadine (Claritin), the antidepressant sertraline (Zoloft), the anti-epileptic lamotrigine (Lamictal), and the inhalation anesthetic isoflurane.[13]
Rachel Carson brought the issue of DDT pesticide toxicity to public awareness with her 1962 book Silent Spring. While many countries have phased out the use of some types of organochlorides such as the US ban on DDT, persistent DDT, PCBs, and other organochloride residues continue to be found in humans and mammals across the planet many years after production and use have been limited. In Arctic areas, particularly high levels are found in marine mammals. These chemicals concentrate in mammals, and are even found in human breast milk. In some species of marine mammals, particularly those that produce milk with a high fat content, males typically have far higher levels, as females reduce their concentration by transfer to their offspring through lactation.[14]
See also
References
- ^ a b Gordon W. Gribble (1998). "Naturally Occurring Organohalogen Compounds". Acc. Chem. Res. 31 (3): 141–152. doi:10.1021/ar9701777.
- ^ Gordon W. Gribble (1999). "The diversity of naturally occurring organobromine compounds". Chemical Society Reviews. 28 (5): 335. doi:10.1039/a900201d.
- ^ Kjeld C. Engvild (1986). "Chlorine-Containing Natural Compounds in Higher Plants". Phytochemistry. 25 (4): 7891–791. doi:10.1016/0031-9422(86)80002-4.
- ^ Gribble, G. W. (1994). "The Natural production of chlorinated compounds". Environmental Science and Technology. 28 (7): 310A–319A. doi:10.1021/es00056a001. PMID 22662801.
- ^ Gribble, G. W. (1996). "Naturally occurring organohalogen compounds - A comprehensive survey". Progress in the Chemistry of Organic Natural Products. 68 (10): 1–423. doi:10.1021/np50088a001. PMID 8795309.
- ^ Public Health Statement - Chloromethane, Centers for Disease Control, Agency for Toxic Substances and Disease Registry
- ^ M. Rossberg et al. "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- ^ a b J R Coats (July 1990). "Mechanisms of toxic action and structure-activity relationships for organochlorine and synthetic pyrethroid insecticides". EHP. 87:. National Center for Biotechnology Information: 255–262. Retrieved 26 February 2015.
{{cite journal}}: CS1 maint: extra punctuation (link) - ^ Robert L. Metcalf "Insect Control" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Wienheim, 2002. doi:10.1002/14356007.a14_263
- ^ Connell, D.; et al. (1999). Introduction to Ecotoxicology. Blackwell Science. p. 68. ISBN 0-632-03852-7.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ Pless, Tanja; Boettger, Michael; Hedden, Peter; Graebe, Jan (1984). "Occurrence of 4-Cl-indoleacetic acid in broad beans and correlation of its levels with seed development". Plant Physiology. 74 (2): 320–3. doi:10.1104/pp.74.2.320. PMC 1066676. PMID 16663416.
- ^ Magnus, Volker; Ozga, Jocelyn A; Reinecke, Dennis M; Pierson, Gerald L; Larue, Thomas A; Cohen, Jerry D; Brenner, Mark L (1997). "4-chloroindole-3-acetic and indole-3-acetic acids in Pisum sativum". Phytochemistry. 46 (4): 675–681. doi:10.1016/S0031-9422(97)00229-X.
- ^ MDL Drug Data Report (MDDR), Elsevier MDL, version 2004.2
- ^ Marine Mammal Medicine, 2001, Dierauf & Gulland