Diazomethane
| |
| |
| Names | |
|---|---|
| IUPAC name
Diazomethane
| |
| Other names
Azimethylene,
Azomethylene, Diazirine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.803 |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH2N2 | |
| Molar mass | 42.04 g/mol |
| Appearance | Yellow gas |
| Odor | musty |
| Density | 1.4 (air=1) |
| Melting point | −145 °C (−229 °F; 128 K) |
| Boiling point | −23 °C (−9 °F; 250 K) |
| hydrolysis[1] | |
| Structure | |
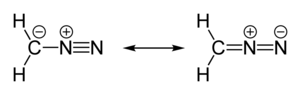
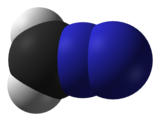
| linear C=N=N | |
| polar | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic and explosive |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
175 ppm (cat, 10 min)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.2 ppm (0.4 mg/m3)[2] |
REL (Recommended)
|
TWA 0.2 ppm (0.4 mg/m3)[2] |
IDLH (Immediate danger)
|
2 ppm[2] |
| Related compounds | |
Related functional groups;
compounds |
R-N=N=N azide), R-N=N-R (azo); R2CN2 R = Ph, tms, CF3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions.[4]
Use
For safety and convenience diazomethane is always prepared as needed as a solution in ether and used as such. It converts carboxylic acids into their methyl esters or into their homologues (see Arndt-Eistert synthesis). In the Büchner–Curtius–Schlotterbeck reaction diazomethane reacts with an aldehyde to form ketones.[5][6]

When diazomethane is reacted with alcohols or phenols in presence of boron trifluoride (BF3), methyl ethers are obtained.
Diazomethane is also frequently used as a carbene source. It readily takes part in 1,3-dipolar cycloadditions.
Preparation

Diazomethane is prepared by hydrolysis of an ethereal solution of an N-methyl nitrosamide with aqueous base. The traditional precursor is N-nitroso-N-methylurea, but this compound is itself somewhat unstable, and nowadays such compounds as N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) and N-methyl-N-nitroso-p-toluenesulfonamide (Diazald) are preferred.[7]
CH2N2 reacts with basic solutions of D2O to give the deuterated derivative CD2N2.[8]
The concentration of CH2N2 can be determined in either of two convenient ways. It can be treated with an excess of benzoic acid in cold Et2O. Unreacted benzoic acid is then back-titrated with standard NaOH. Alternatively, the concentration of CH2N2 in Et2O can be determined spectrophotometrically at 410 nm where its extinction coefficient, ε, is 7.2.[citation needed] The gas-phase concentration of diazomethane can be determined using photoacoustic spectroscopy.[4]
Related compounds
Many substituted derivatives of diazomethane have been prepared:
- The very stable (CF3)2CN2 (2-diazo-1,1,1,3,3,3-hexafluoropropane; b.p. 12–13 °C),[9]
- Ph2CN2 (diazo(diphenyl)methane; m.p. 29–30 °C).[10]
- (CH3)3SiCHN2 (trimethylsilyldiazomethane), which is commercially available as a solution and is as effective as CH2N2 for methylation.[11]
- PhC(H)N2, a red liquid b.p.< 25 °C at 0.1 mm Hg.[12]
Safety
Diazomethane is toxic by inhalation or by contact with the skin or eyes (TLV 0.2ppm). Symptoms include chest discomfort, headache, weakness and, in severe cases, collapse.[13] Symptoms may be delayed. Deaths from diazomethane poisoning have been reported. In one instance a laboratory worker consumed a hamburger near a fumehood where he was generating a large quantity of diazomethane, and died four days later from fulminating pneumonia.[14] Like any other alkylating agent it is expected to be carcinogenic, but such concerns are overshadowed by its serious acute toxicity.
CH2N2 may explode in contact with sharp edges, such as ground-glass joints, even scratches in glassware.[citation needed] Glassware should be inspected before use and preparation should take place behind a blast shield. Specialized kits to prepare diazomethane with flame-polished joints are commercially available.
The compound explodes when heated beyond 100 °C, exposed to intense light, alkali metals, or calcium sulfate. Use of a blast shield is highly recommended while using this compound.
References
- ^ ICSC 1256 - DIAZOMETHANE
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0182". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Diazomethane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Proctor, Lee D.; Warr, Antony J. (November 2002). "Development of a Continuous Process for the Industrial Generation of Diazomethane". Organic Process Research & Development. 6 (6): 884–892. doi:10.1021/op020049k.
- ^ Buchner, E.; Curtius, Th. (1885). "Synthese von Ketonsäureäthern aus Aldehyden und Diazoessigäther". Berichte der Deutschen Chemischen Gesellschaft. 18: 2371–2377. doi:10.1002/cber.188501802118.
- ^ Schlotterbeck, F. (1907). "The conversion of aldehydes and ketones through diazomethane". Berichte der Deutschen Chemischen Gesellschaft. 40: 479–483. doi:10.1002/cber.19070400179.
- ^ Reed, Donald E.; James A. Moore (1961). "DIAZOMETHANE". Organic Syntheses. 41: 16. doi:10.15227/orgsyn.041.0016.
- ^ P. G. Gassman and W. J. Greenlee (1988). "Dideuterodiazomethane". Organic Syntheses; Collected Volumes, vol. 6, p. 432.
- ^ W. J. Middleton; D. M. Gale (1988). "Bis(Trifluoromethyl)diazomethane". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 6, p. 161. - ^ L. I. Smith, K. L. Howard (1955). "Diphenyldiazomethane"". Organic Syntheses; Collected Volumes, vol. 3, p. 351.
- ^ T. Shioiri, T. Aoyama, S. Mori. "Trimethylsilyldiazomethane". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 8, p. 612. - ^ X. Creary (1990). "Tosylhydrazone Salt Pyrolyses: Phenydiazomethanes". Organic Syntheses; Collected Volumes, vol. 7, p. 438.
- ^ Muir, GD (ed.) 1971, Hazards in the Chemical Laboratory, The Royal Institute of Chemistry, London.
- ^ LeWinn, E.B. "Diazomethane Poisoning: Report of a fatal case with autopsy", The American Journal of the Medical Sciences, 1949, 218, 556-562.
External links
- MSDS diazomethane
- CDC - NIOSH Pocket Guide to Chemical Hazards
- Sigmaaldrich technical bulletin (PDF)
- Sigma-Aldrich diazomethane applications and commercial availability of (Diazald) precursor
- The Buchner–Curtius–Schlotterbeck reaction @ Institute of Chemistry, Skopje, Macedonia
- Identification of Artifacts (By-Products) in Diazomethane and Trimethylsilyldiazomethane Reactions


