Porphobilinogen
Appearance
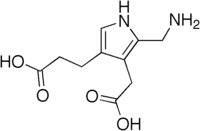
| |
| Names | |
|---|---|
| IUPAC name
3-[5-(Aminomethyl)-4-(carboxymethyl)-1H-pyrrol-3-yl]propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.970 |
| EC Number |
|
| MeSH | Porphobilinogen |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H14N2O4 | |
| Molar mass | 226.229 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Porphobilinogen (PBG) is a pyrrole involved in porphyrin metabolism.
It is generated from aminolevulinate (ALA) by the enzyme ALA dehydratase. PBG is then converted into hydroxymethyl bilane by the enzyme porphobilinogen deaminase, also known as hydroxymethylbilane synthase.
Acute intermittent porphyria causes an increase in urinary porphobilinogen.[1]
References
- ^ Aarsand, AK; Petersen PH; Sandberg S (April 2006). "Estimation and application of biological variation of urinary delta-aminolevulinic acid and porphobilinogen in healthy individuals and in patients with acute intermittent porphyria". Clinical Chemistry. 52 (4): 650–656. doi:10.1373/clinchem.2005.060772. PMID 16595824.
