Paraffin wax


Paraffin wax is a white or colourless soft solid, derivable from petroleum, coal or oil shale, that consists of a mixture of hydrocarbon molecules containing between twenty and forty carbon atoms. It is solid at room temperature and begins to melt above approximately 37 °C (99 °F);[1] its boiling point is >370 °C (698 °F).[2] Common applications for paraffin wax include lubrication, electrical insulation, and candles; [3] dyed paraffin wax can be made into crayons. It is distinct from kerosene, another petroleum product that is sometimes called paraffin.
Un-dyed, unscented paraffin candles are odorless and bluish-white. Paraffin wax was first created in 1830 in Germany, and marked a major advancement in candlemaking technology, as it burned more cleanly and reliably than tallow candles and was cheaper to produce.[4]
In chemistry, paraffin is used synonymously with alkane, indicating hydrocarbons with the general formula CnH2n+2. The name is derived from Latin parum ("barely") + affinis, meaning "lacking affinity" or "lacking reactivity", referring to paraffin's unreactive nature.[5]
Properties
Paraffin wax is mostly found as a white, odorless, tasteless, waxy solid, with a typical melting point between about 46 and 68 °C (115 and 154 °F),[6] and a density of around 900 kg/m3.[7] It is insoluble in water, but soluble in ether, benzene, and certain esters. Paraffin is unaffected by most common chemical reagents but burns readily.[8] Its heat of combustion is 42 MJ/kg.
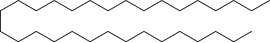
The hydrocarbon C31H64 is a typical component of paraffin wax.
Paraffin wax is an excellent electrical insulator, with a resistivity of between 1013 and 1017 ohm metre.[9] This is better than nearly all other materials except some plastics (notably Teflon). It is an effective neutron moderator and was used in James Chadwick's 1932 experiments to identify the neutron.[10][11]
Paraffin wax is an excellent material for storing heat, with a specific heat capacity of 2.14–2.9 J g−1 K−1 (joules per gram kelvin) and a heat of fusion of 200–220 J g−1.[12] This property is exploited in modified drywall for home building material: a certain type of wax (with the right melting point) is infused in the drywall during manufacture so that it melts during the day, absorbing heat, and solidifies again at night, releasing the heat.[13] Paraffin wax phase-change cooling coupled with retractable radiators was used to cool the electronics of the Lunar Rover.[14] Wax expands considerably when it melts and this allows its use in wax element thermostats for industrial, domestic and, particularly, automobile purposes.[15][16]
History
Paraffin wax was first created in 1830 by the German chemist Karl von Reichenbach when he tried to develop the means to efficiently separate and refine the waxy substances naturally occurring in petroleum. Paraffin represented a major advance in the candlemaking industry because it burned more cleanly and reliably, and was cheaper to manufacture than any other candle fuel. Paraffin wax initially suffered from a low melting point; however, this shortcoming was later remedied by the addition of harder stearic acid. The production of paraffin wax enjoyed a boom in the early 20th century as a result of the growth of the meatpacking and oil industries, which created paraffin and stearic acid as byproducts.[4]
Manufacturing
The feedstock for paraffin is slack wax, which is a mixture of oil and wax, a byproduct from the refining of lubricating oil.
The first step in making paraffin wax is to remove the oil (de-oiling or de-waxing) from the slack wax. The oil is separated through crystallization. Most commonly, the slack wax is heated, mixed with one or more solvents such as a ketone and then cooled. As it cools, wax crystallizes out of the solution, leaving only oil. This mixture is filtered into two streams: solid (wax plus some solvent) and liquid (oil and solvent). After the solvent is recovered by distillation, the resulting products are called "product wax" (or "press wax") and "foots oil". The lower the percentage of oil in the wax, the more refined it is considered (semi-refined versus fully refined).[17] The product wax may be further processed to remove colors and odors. The wax may finally be blended together to give certain desired properties such as melt point and penetration. Paraffin wax is sold in either liquid or solid form.[18][19][20]
Applications
In industrial applications, it is often useful to modify the crystal properties of the paraffin wax, typically by adding branching to the existing carbon backbone chain. The modification is usually done with additives, such as EVA copolymers, microcrystalline wax, or forms of polyethylene. The branched properties result in a modified paraffin with a higher viscosity, smaller crystalline structure, and modified functional properties. Pure paraffin wax is rarely used for carving original models for casting metal and other materials in the lost wax process, as it is relatively brittle at room temperature and presents the risks of chipping and breakage when worked. Soft and pliable waxes, like beeswax, may be preferred for such sculpture, but "investment casting waxes," often paraffin-based, are expressly formulated for the purpose.
In a pathology laboratory, paraffin wax is used to impregnate tissue prior to sectioning thin samples of tissue. Water is removed from tissue through ascending strengths of alcohol (75% to absolute) and the tissue is cleared in an organic solvent such as xylene. The tissue is then placed in paraffin wax for a number of hours and then set in a mold with wax to cool and solidify; sections are cut then on a microtome.
Other uses
- Candle-making
- Coatings for waxed paper or cloth
- Food-grade paraffin wax:
- Shiny coating used in candy-making; although edible, it is nondigestible, passing right through the body without being broken down
- Coating for many kinds of hard cheese, like Edam cheese
- Sealant for jars, cans, and bottles
- Chewing gum additive
- Investment casting
- Anti-caking agent, moisture repellent, and dustbinding coatings for fertilizers
- Agent for preparation of specimens for histology
- Bullet lubricant – with other ingredients, such as olive oil and beeswax
- Phlegmatizing agent, commonly used to stabilise/desensitize high explosives such as RDX
- Crayons
- Solid propellant for hybrid rocket motors[21]
- Component of surfwax, used for grip on surfboards in surfing
- Component of glide wax, used on skis and snowboards
- Friction-reducer, for use on handrails and cement ledges, commonly used in skateboarding
- Ink. Used as the basis for solid ink different color blocks of wax for thermal printers. The wax is melted and then sprayed on the paper producing images with a shiny surface
- Microwax:[22] food additive, a glazing agent with E number E905
- Forensic investigations: the nitrate test uses paraffin wax to detect nitrates and nitrites on the hand of a shooting suspect
- Antiozonant agents: blends of paraffin and micro waxes are used in rubber compounds to prevent cracking of the rubber; the admixture of wax migrates to the surface of the product and forms a protective layer. The layer can also act as a release agent, helping the product separate from its mould.[23]
- Mechanical thermostats and actuators, as an expansion medium for activating such devices[16]
- "Potting" guitar pickups, which reduces microphonic feedback caused from the subtle movements of the pole pieces
- "Potting" of local oscillator coils to prevent microphonic frequency modulation in low end FM radios.
- Texitle manufacturing processes, such as that used for Eisengarn thread.
- Wax baths for beauty and therapy purposes
- Thickening agent in many paintballs
- Moisturiser in toiletries and cosmetics such as Vaseline, though potentially comedogenic.
- Prevents oxidation on the surface of polished steel and iron[24]
- Phase change material for thermal energy storage
- Manufacture of boiled leather armor and books
- Skateboard wax
- Paraffin microactuator[26]
- Neutron radiation shielding
- waterproofing agent for waxed cotton garments and commercially important in the early water proofing of ship sails.
Occupational safety
People can be exposed to paraffin in the workplace by breathing it in, skin contact, and eye contact. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) for paraffin wax fume exposure of 2 mg/m3 over an 8-hour workday.[27]
See also
References
- ^ Freund, Mihály; Mózes, Gyula (1982). Paraffin products: properties, technologies, applications. Translated by Jakab, E. Amsterdam, Netherlands: Elsevier. p. 121. ISBN 0-444-99712-1.
{{cite book}}: Invalid|ref=harv(help) - ^ "Paraffin Wax". Chemical book. Retrieved 25 October 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Raw materials and candles production processes, AECM
- ^ a b "History of Candles". National Candle Association. Retrieved 25 February 2016.
- ^ "Paraffin, n". Oxford English Dictionary. Oxford, England: Oxford University Press. March 2009.
- ^ Nasser, William E (1999). "Waxes, Natural and Synthetic". In McKetta, John J (ed.). Encyclopedia of Chemical Processing and Design. Vol. 67. New York: Marcel Dekker. p. 17. ISBN 0-8247-2618-9. This can vary widely, even outside the quoted range, according to such factors as oil content and crystalline structure.
- ^ Kaye, George William Clarkson; Laby,Thomas Howell. "Mechanical properties of materials". Kaye and Laby Tables of Physical and Chemical Constants. National Physical Laboratory. Retrieved 25 October 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Seager, Spencer L.; Slabaugh, Michael. "Alkane reactions". Chemistry for Today: General, Organic, and Biochemistry. Belmont, CA: Cengage. p. 364. ISBN 978-0-538-73332-8.
- ^ "Electrical insulating materials". Kaye and Laby Tables of Physical and Chemical Constants. National Physical Laboratory. 1995. Retrieved 25 October 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Attenuation of fast neutrons: neutron moderation and diffusion". Kaye and Laby Tables of Physical and Chemical Constants. National Physical Laboratory. Retrieved 25 October 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Rhodes, Richard (1981). The Making of the Atomic Bomb. New York: Simon and Schuster. p. 163. ISBN 0-671-44133-7.
- ^ "Specific Heat Capacity". Diracdelta.co.uk Science and Engineering Encyclopedia. Dirac Delta Consultants Ltd, Warwick, England. Retrieved 25 October 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Micronal PCM SmartBoard".
- ^ Dean, W. G.; Karu, Z. S. (February 1993). "Space Station thermal storage/refrigeration system research and development". Final Report Lockheed Missiles and Space Co. NASA. Bibcode:1993lock.rept.....D.
- ^ Wax-pellet thermostat United States Patent 4948043
- ^ a b
Bodén, Roger. "Paraffin Microactuator" (pdf). Materials Science Sensors and Actuators. University of Uppsala. Retrieved 25 October 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Paraffin Wax (Fully Refined)". Barasat Wax Refiner. Retrieved 21 December 2012.
- ^ "Wax Refining". The International Group, Inc. Retrieved 21 December 2012.
- ^ "Paraffin wax". Bitumen Engineering. Retrieved 21 December 2012.
- ^ "Manufacturing Process". Barasat Wax Refiner. Retrieved 21 December 2012.
- ^ Staff (Fall 2004). "Rocket motor uses common household product for fuel" (PDF). OASIS Ocean Air Space Industry Site. 1 (3). Stennis Space Center Pearlington, MS: NASA: 6. Retrieved 28 November 2008.
- ^ "Paraffin, microcrystalline, petrolatum, wax blends - Microcrystalline Wax". igiwax.com. Retrieved 29 April 2017.
- ^ (Freund & Mózes 1982, p. 272)
- ^
Dick, William B (1872). "Encyclopedia Of Practical Receipts And Processes". New York: Dick and Fitzgerald. Retrieved 25 October 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Instrument Information". NASA. 2007. Retrieved 24 January 2017.
- ^ Ogden, Sam; Klintberg, Lena; Thornell, Greger; Hjort, Klas; Bodén, Roger (30 November 2013). "Review on miniaturized paraffin phase change actuators, valves, and pumps". Microfluidics and Nanofluidics. doi:10.1007/s10404-013-1289-3.
- ^ "CDC - NIOSH Pocket Guide to Chemical Hazards - Paraffin wax fume". cdc.gov. Retrieved 27 November 2015.

