Silicone

Silicones, or polysiloxanes, are inorganic-organic polymers with the chemical formula [R2SiO]n, where R = organic groups such as methyl, ethyl, and phenyl. These materials consist of an inorganic silicon-oxygen backbone (...-Si-O-Si-O-Si-O-...) with organic side groups attached to the silicon atoms, which are four-coordinate. In some cases organic side groups can be used to link two or more of these -Si-O- backbones together. By varying the -Si-O- chain lengths, side groups, and crosslinking, silicones can be synthesized with a wide variety of properties and compositions. They can vary in consistency from liquid to gel to rubber to hard plastic. The most common type is linear polydimethylsiloxane or PDMS. The second largest group of silicone materials is based on silicone resins, which are formed by branched and cage-like oligosiloxanes.


Chemical terminology
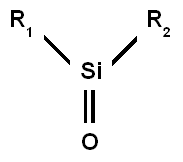
Silicone is often mistakenly referred to as "silicon". Although silicones contain silicon atoms, they are not made up exclusively of silicon, and have completely different physical characteristics from elemental silicon.
The word "silicone" is derived from ketone. Dimethylsilicone and dimethyl ketone (a.k.a. acetone) have analogous formulas, thus it was surmised incorrectly that they have analogous structures. The same terminology is used for compounds such as silane, which is an analogue of methane). A true silicone group with a double bond between oxygen and silicon do not exist (see figure), Polysiloxanes are called "silicone" due to early mistaken assumptions about structure.
Safety
Controversy developed during the 1990s around allegations that the silicone gel in breast implants was responsible for several health problems, including autoimmune diseases. Systemic reviews were undertaken by governments in a number of countries including Canada (Canadian Expert Advisory Committee review, 1992), Germany, France (ANDEM, 1996), USA (US National Science Panel, 1997-8, UK Independent Review Group 1998, and the U.S. Institute of Medicine, 1999), and the Scientific Technical Opinions Assessment (STOA) report commissioned by the European Parliament in 2001 (updated in 2003). A clear consensus has emerged from these independent scientific reviews that, to date, there is no evidence of a causal link between the implantation of silicones and connective tissue disease.[citation needed]
Firestops


Silicone foams have been used in North American as well as the Israeli Dimona nuclear reactor buildings in an attempt to firestop openings within fire-resistance rated wall and floor assemblies to prevent the spread of flames and smoke from one room to another. (The Israelis wisely switched to the somewhat more expensive yet much safer "elastomer" version of this product, which avoids most safety concerns associated with the foamed version.) Silicone foam firestops have been the subject of serious controversy and press attention due to lack of proper bounding and smoke development due to the pyrolysis of combustible components within the foam, hydrogen gas escape, shrinkage and cracking. When properly installed, silicone foam firestops can be fabricated for building code compliance. Advantages include flexibility and high dielectric strength. Disadvantages include poor bounding, combustibility (hard to extinguish) and significant smoke development, which has led to many reportable events for the US Nuclear Regulatory Commission, to ensure proper installations in facilities under their jurisdiction.
Sealants in building construction and maintenance
One-part silicone sealants are in common use to seal gaps, joints and crevices in buildings. Silicones are abundantly available for this purpose, in professional, as well as retail grades. One-part silicones cure by absorbing atmospheric moisture, which helps in the professional installation. To make a very smooth silicone seal, apart from masking the edges with tape, if practical, professional caulkers often wet wooden spoons and assorted, hand-crafted wooden tools, which they soak in water with diluted dishsoap. The silicone will not stick to wet, soapy wood, which makes this ideal for creating a perfectly smooth surface. Do-it-yourselfers typically use a moistened finger to trace neat beads into silicone caulking.
Similar methods work for urethane caulking, against which silicones compete quite heavily. White silicones can, at times, turn slightly yellow over time.
The strength and reliability of silicone rubber is widely acknowledged in the construction industry. Automotive body manufacturing plants and paint shops must avoid the presence of all silicones, as a mere hint of its presence in any form can cause severe failures in automotive paints. Vendors and contractors in such plants are often requested to verify in writing that they will not bring any silicones into the plant.
In the plumbing and automotive fields, silicone grease is often used as a lubricant. In plumbing, the grease is typically applied to O-rings in faucets and valves - for example, all official Moen replacement cartridges are pre-lubricated with it. In the automotive field, silicone grease is typically used as a lubricant for brake components since it is stable at high temperatures, is not water-soluble, and is far less likely to foul brake pads.
Cooking Applications
Silicone is also impregnated into parchment paper and used as a non-stick material for applications such as baking and steaming. The silicone also makes the paper heat- and grease-resistant. This allows the paper to line cookie sheets and act as a replacement for greasing, thereby speeding mass production of baked goods. It is also commonly used in pouch cooking, where ingredients are sealed into a container made of parchment paper and allowed to steam.
Silicone rubber is also used to make utensils (notably spatulas) and bakeware.
Electronic Components
Electronic components sometimes are protected from environmental influence by enclosing them in silicone. This increases the stability against mechanical shock, radiation, vibration and especially the electrical insulation. Therefore it is used for components for high and longlasting performance under hard conditions like in space (satellite technology).
See also
- Breast implant
- Bounding
- Firestop
- Injection Molding of Liquid Silicone Rubber
- Negev Nuclear Research Center
- Neuticles (Animal testicular implants)
- Nuclear Reactor
- Nuclear Regulatory Commission
- Parchment
- Passive fire protection
External links
- NIRS Reactorwatch
- ccnr.org Representative Ed Markey's Statements concerning flammable firestops
- Garden State EnviroNet Statement on NRC Silicone Foam Issues
- USNRC Information Notice 88-56
- Silicone Polymers (Virtual Chembook, Elmhurst College)
- Science of Silicone Polymers (Silicone Science On-line, Centre Européen des Silicones - CES)
- Silicon Chemistry (Silicone Chemistry Basics, Dow Corning)
- Injection Molding of Liquid Silicone Rubber(SIMTEC Silicone Parts LLC)
- Damp Proof Course Information
