Gram-negative bacteria

Gram-negative bacteria are a class of bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation,[1] making positive identification possible. The thin peptidoglycan layer of their cell wall is sandwiched between an inner cell membrane and a bacterial outer membrane. In Gram staining, the outer lipid-based membrane of gram-negative bacteria is removed by an alcohol solution. The alcohol also decolorizes the then exposed peptidoglycan layer by dissolving away the previously applied crystal violet. A counterstain (safranin or fuchsine) is then added which recolorizes the bacteria red or pink.
Gram-positive bacteria on the other hand have a thicker peptidoglycan layer in their cell wall outside the cell membrane, which retains the crystal violet stain during the alcohol wash, so long as it is timed correctly. The counter stain may also be absorbed by gram-positive bacteria but the darker crystal violet stain predominates visually.
Characteristics

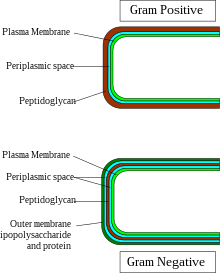
Gram-negative bacteria display Template:J:
- Cell membrane (cytoplasmic).
- Thin peptidoglycan layer (which is much thicker in gram-positive bacteria)
- Outer membrane containing lipopolysaccharide (LPS, which consists of lipid A, core polysaccharide, and O antigen) in its outer leaflet and phospholipids in the inner leaflet
- Porins exist in the outer membrane, which act like pores for particular molecules
- There is a space between the peptidoglycan layer and the secondary cell membrane called the periplasmic space
- The S-layer is directly attached to the outer membrane rather than the peptidoglycan
- If present, flagella have four supporting rings instead of two
- No teichoic acids or lipoteichoic acids are present
- Lipoproteins are attached to the polysaccharide backbone.
- Some of them contain Braun's lipoprotein, which serves as a link between the outer membrane and the peptidoglycan chain by a covalent bond
- Most, with very few exceptions, do not form spores.
- Release some endotoxin
Classification
Along with cell shape, Gram staining is a rapid diagnostic tool and once was used to group species at the subdivision of Bacteria.

Historically, the kingdom Monera was divided into four divisions based on Gram staining: Firmacutes (+), Gracillicutes (−), Mollicutes (0) and Mendocutes (var.).[2]
Since 1987, the monophyly of the gram-negative bacteria has been disproven with molecular studies.[3] However some authors, such as Cavalier-Smith still treat them as a monophyletic clade and refer to the group as subkingdom "Negibacteria".[4]
Outer cell membrane bacterial classification
This section may be too technical for most readers to understand. (March 2014) |
Though bacteria are traditionally divided into the two main groups of gram-positive and gram-negative, based on their Gram stain retention, this classification system can be ambiguous; it can refer to three different aspects, those of staining result, cell-envelope organisation, or taxonomic group, which do not necessarily coalesce for some bacterial species.[5][6][7][8] The staining response is also not a reliable phylogenetic character as these two kinds of bacteria do not form phylogenetically coherent groups.[5] However, Gram staining response of bacteria is an empirical criterion; its basis lies in the marked differences in the ultrastructure and chemical composition of the two main kinds of prokaryotic cells that are found in nature. These two kinds of cells are distinguished from each other based upon the presence or absence of an outer lipid membrane, which is a reliable and fundamental characteristic of bacterial cells.[5][9] All gram-positive bacteria are bounded by only a single unit lipid membrane and they generally contain a thick layer (20–80 nm) of peptidoglycan responsible for retaining the Gram stain. A number of other bacteria that are bounded by a single membrane, but stain gram-negative due to either lack of the peptidoglycan layer (viz., mycoplasmas) or their inability to retain the Gram stain because of their cell wall composition, also show close relationship to the gram-positive bacteria. For the bacterial (prokaryotic) cells that are bounded by a single cell membrane, the term Monoderm Bacteria or Monoderm Prokaryotes has been proposed.[5][5][9] In contrast to gram-positive bacteria, all archetypical gram-negative bacteria are bounded by both a cytoplasmic membrane and an outer cell membrane, and they contain only a thin layer of peptidoglycan (2–3 nm) in between these two membranes. The presence of both inner and outer cell membranes defines a new compartment in these cells, the periplasmic space or the periplasmic compartment. These bacteria/prokaryotes have been designated as Diderm Bacteria.[5][5][9] The distinction between the monoderm and diderm prokaryotes is also supported by conserved signature indels in a number of important proteins (viz. DnaK, GroEL).[5][6][9][10] Of these two structurally distinct groups of prokaryotic organisms, monoderm prokaryotes are indicated to be ancestral. Based upon a number of different observations including that the gram-positive bacteria are the major producers of antibiotics and that gram-negative bacteria are, in general, resistant to them, it has been proposed that the outer cell membrane in gram-negative bacteria (diderms) evolved as a protective mechanism against antibiotic selection pressure.[5][6][9][10] Some bacteria such as Deinococcus, which stain gram-positive due to the presence of a thick peptidoglycan layer, but also possess an outer cell membrane are suggested as intermediates in the transition between monoderm (gram-positive) and diderm (gram-negative) bacteria.[5][10] The diderm bacteria can also be further differentiated between simple diderms lacking lipopolysaccharide, the archetypical diderm bacteria, in which the outer cell membrane contains lipopolysaccharide, and the diderm bacteria, in which outer cell membrane is made up of mycolic acid.[7][8][10][11]
In addition, a number of bacterial taxa (viz. Negativicutes, Fusobacteria, Synergistetes, and Elusimicrobia) that are either part of the phylum Firmicutes or branches in its proximity are also found to possess a diderm cell structure.[8][10][11] However, a conserved signature indel (CSI) in the HSP60 (GroEL) protein distinguishes all traditional phyla of gram-negative bacteria (e.g., Proteobacteria, Aquificae, Chlamydiae, Bacteroidetes, Chlorobi, Cyanobacteria, Fibrobacteres, Verrucomicrobia, Planctomycetes, Spirochetes, Acidobacteria) from these other atypical diderm bacteria as well as other phyla of monoderm bacteria (e.g., Actinobacteria, Firmicutes, Thermotogae, Chloroflexi).[10] The presence of this CSI in all sequenced species of conventional LPS-containing gram-negative bacterial phyla provides evidence that these phyla of bacteria form a monophyletic clade and that no loss of the outer membrane from any species from this group has occurred.[10]
Example species
The proteobacteria are a major group of gram-negative bacteria, including Escherichia coli (E. coli), Salmonella, Shigella, and other Enterobacteriaceae, Pseudomonas, Moraxella, Helicobacter, Stenotrophomonas, Bdellovibrio, acetic acid bacteria, Legionella etc. Other notable groups of gram-negative bacteria include the cyanobacteria, spirochaetes, green sulfur, and green non-sulfur bacteria.
Medically relevant gram-negative cocci include the three organisms that cause a sexually transmitted disease (Neisseria gonorrhoeae), a meningitis (Neisseria meningitidis), and respiratory symptoms (Moraxella catarrhalis).
Medically relevant gram-negative bacilli include a multitude of species. Some of them cause primarily respiratory problems (Hemophilus influenzae, Klebsiella pneumoniae, Legionella pneumophila, Pseudomonas aeruginosa), primarily urinary problems (Escherichia coli, Proteus mirabilis, Enterobacter cloacae, Serratia marcescens), and primarily gastrointestinal problems (Helicobacter pylori, Salmonella enteritidis, Salmonella typhi).
Gram-negative bacteria associated with hospital-acquired infections include Acinetobacter baumannii, which cause bacteremia, secondary meningitis, and ventilator-associated pneumonia in hospital intensive-care units.
Medical treatment
This section needs additional citations for verification. (April 2013) |
One of the several unique characteristics of gram-negative bacteria is the structure of the outer membrane. The outer leaflet of the membrane comprises a complex lipopolysaccharide (LPS) whose lipid portion acts as an endotoxin. If LPS enters the circulatory system, it causes a toxic reaction, with the sufferer developing a high temperature, high respiration rate, and low blood pressure. This may lead to endotoxic shock, which can be fatal.
This outer membrane protects the bacteria from several antibiotics, dyes, and detergents that would normally damage either the inner membrane or the cell wall's (peptidoglycan). The outer membrane provides these bacteria with resistance to lysozyme and penicillin. However, alternative medicinal treatments such as lysozyme with EDTA and the antibiotic ampicillin have been developed to combat the protective outer membrane of some pathogenic gram-negative organisms. Other drugs can also be used, significant ones being chloramphenicol, streptomycin, and nalidixic acid.
The pathogenic capability of gram-negative bacteria is often associated with certain components of their membrane, in particular, the lipopolysaccharide layer (also known as the LPS or endotoxin layer).[1] In humans, the presence of LPS triggers an innate immune response, activating the immune system and producing cytokines (hormonal regulators). Inflammation is a common reaction to cytokine production, which can also produce host toxicity. The innate immune response to LPS, however, is not synonymous with pathogenicity, or the ability to cause disease. In fact, the innate immune response is triggered purely by LPS.
Orthographic note
The adjectives gram-positive and gram-negative are named after Hans Christian Gram; as eponymous adjectives, they are conventionally written in lowercase.[12][13][14]
See also
References
 This article incorporates public domain material from Science Primer. NCBI. Archived from the original on 2009-12-08.
This article incorporates public domain material from Science Primer. NCBI. Archived from the original on 2009-12-08.
- Notes
- ^ a b Baron S, Salton MRJ, Kim KS (1996). "Structure". In Baron S; et al. (eds.). Baron's Medical Microbiology (4th ed.). Univ of Texas Medical Branch. ISBN 0-9631172-1-1. PMID 21413343.
{{cite book}}: Explicit use of et al. in:|editor=(help)CS1 maint: multiple names: authors list (link) - ^ Gibbons, N. E.; Murray, R. G. E. (1978). "Proposals Concerning the Higher Taxa of Bacteria". International Journal of Systematic Bacteriology. 28 (1): 1–6. doi:10.1099/00207713-28-1-1.
- ^ Woese CR (June 1987). "Bacterial evolution". Microbiol. Rev. 51 (2): 221–71. PMC 373105. PMID 2439888.
- ^ Cavalier-Smith T (2006). "Rooting the tree of life by transition analyses". Biol. Direct. 1: 19. doi:10.1186/1745-6150-1-19. PMC 1586193. PMID 16834776.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e f g h i j Gupta RS (December 1998). "Protein phylogenies and signature sequences: A reappraisal of evolutionary relationships among archaebacteria, eubacteria, and eukaryotes". Microbiol. Mol. Biol. Rev. 62 (4): 1435–91. PMC 98952. PMID 9841678.
- ^ a b c Gupta RS (2000). "The natural evolutionary relationships among prokaryotes". Crit. Rev. Microbiol. 26 (2): 111–31. doi:10.1080/10408410091154219. PMID 10890353.
- ^ a b Desvaux M, Hébraud M, Talon R, Henderson IR (April 2009). "Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue". Trends Microbiol. 17 (4): 139–45. doi:10.1016/j.tim.2009.01.004. PMID 19299134.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Sutcliffe IC (October 2010). "A phylum level perspective on bacterial cell envelope architecture". Trends Microbiol. 18 (10): 464–70. doi:10.1016/j.tim.2010.06.005. PMID 20637628.
- ^ a b c d e Gupta RS (August 1998). "What are archaebacteria: life's third domain or monoderm prokaryotes related to gram-positive bacteria? A new proposal for the classification of prokaryotic organisms". Mol. Microbiol. 29 (3): 695–707. doi:10.1046/j.1365-2958.1998.00978.x. PMID 9723910.
- ^ a b c d e f g Gupta RS (August 2011). "Origin of diderm (gram-negative) bacteria: antibiotic selection pressure rather than endosymbiosis likely led to the evolution of bacterial cells with two membranes". Antonie Van Leeuwenhoek. 100 (2): 171–82. doi:10.1007/s10482-011-9616-8. PMC 3133647. PMID 21717204.
- ^ a b Marchandin H, Teyssier C, Campos J, Jean-Pierre H, Roger F, Gay B, Carlier JP, Jumas-Bilak E (June 2010). "Negativicoccus succinicivorans gen. nov., sp. nov., --~~~~isolated from human clinical samples, emended description of the family Veillonellaceae any classis nov., Selenomonadales ord. nov. and Acidaminococcaceae fam. nov. in the bacterial phylum Firmicutes". Int. J. Syst. Evol. Microbiol. 60 (Pt 6): 1271–9. doi:10.1099/ijs.0.013102-0. PMID 19667386.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Centers for Disease Control and Prevention. Emerging Infectious Diseases Journal Style Guide. Preferred Usage
- ^ Merriam-Webster, Merriam-Webster's Medical Dictionary, Merriam-Webster.
- ^ Elsevier, Dorland's Illustrated Medical Dictionary, Elsevier.
