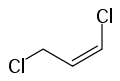
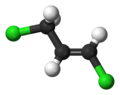
1,3-Dichloropropene
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3-Dichloroprop-1-ene | |||
| Other names
AQL Agrocelhone, DD92, 1,3-D, Dorlone, Nematox, Telone, Nemex, cis-Dichloropropene, Di-Trapex CP, Vorlex 201, dichloro-1,3-propene, 1,3-dichloro-1-propene, 1,3-dichloro-2-propene, alpha-chloroallylchloride, chloroallylchloride, gamma-chloroallylchloride, chloroallyl chloride, chloroorpropenyl chloride, 1,3-dichloropropylene, 3-D, DCP, 3-Chloroallyl chloride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.024 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | 1,3-dichloro-1-propene | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H4Cl2 | |||
| Molar mass | 110.97 g/mol | ||
| Appearance | Colorless to straw-colored liquid | ||
| Odor | sweet, chloroform-like | ||
| Density | 1.217 g/mL (cis); 1.224 g/mL (trans) | ||
| Melting point | −84.5 °C (−120.1 °F; 188.7 K) | ||
| Boiling point | 104 °C (219 °F; 377 K) (cis); 112 °C (trans) | ||
| 2.18 g/L (cis) @ 25 °C; 2.32 g/L (trans) @ 25 °C | |||
| log P | 1.82 | ||
| Vapor pressure | 34.4 mm Hg @ 25 °C (cis); 23.0 mm Hg @ 25 °C (trans) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 28 °C (82 °F; 301 K) | ||
| > 500 °C (932 °F; 773 K) | |||
| Explosive limits | 5.3% - 14.5% (80 °C) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[1] | ||
REL (Recommended)
|
Ca TWA 1 ppm (5 mg/m3) [skin][1] | ||
IDLH (Immediate danger)
|
Ca [N.D.][1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
1,3-Dichloropropene, sold under diverse trade names, is an organochlorine compound. It is colorless liquid with a sweet smell. It dissolves in water and evaporates easily. It is used mainly in farming as a pesticide, specifically as a preplant fumigant and nematicide. It is widely used in the US and other countries, but is in the process of being phased out in the European Union.[2]
Production, chemical properties, biodegradation
It is a byproduct in the chlorination of propene to make allyl chloride.[3]
It is usually obtained as a mixture of the geometric isomers, called Z-1,3-dichloropropene, and E-1,3-dichloropropene. Although it was first applied in agriculture in the 1950s, at least two biodegradation pathways have evolved. One pathway degrades the chlorocarbon to acetaldehyde via chloroacrylic acid.[4]
Safety
The TLV-TWA for 1,3-dichloropropene (DCP) is 1 ppm.[5] It is a contact irritant. A wide range of complications have been reported.[6]
Carcinogenicity
Evidence for the carcinogenicity of 1,3-dichloropropene in humans is inadequate, but results from several cancer bioassays provide adequate evidence of carcinogenicity in animals. In the US, the Department of Health and Human Services (DHHS) has determined that 1,3-dichloropropene may reasonably be anticipated to be a carcinogen. The International Agency for Research on Cancer (IARC) has determined that 1,3-dichloropropene is possibly carcinogenic to humans. The EPA has classified 1,3-dichloropropene as a probable human carcinogen.[6]
Use
1,3-Dichloropropene is used as a pesticide in the following crops: [3]
| Crop | Pounds (lb) | Primary Pesticide? |
|---|---|---|
| Tobacco | 12,114,887 | Yes |
| Potatoes | 12,044,736 | Yes |
| Sugar Beets | 5,799,613 | Yes |
| Cotton | 3,735,543 | Yes |
| Peanuts | 3,463,003 | Yes |
| Sweet Potatoes | 1,210,872 | Yes |
| Onions | 674,183 | Yes |
| Carrots | 531,752 | Yes |
| Watermelons | 133,801 | No |
| Cantaloups | 121,395 | No |
| Cucumbers | 76,735 | No |
| Strawberries | 71,753 | No |
| Sweet Peppers | 28,247 | No |
| Melons | 12,471 | No |
| Blueberries | 3,090 | No |
| Asparagus | 1,105 | No |
Contamination
The ATSDR has extensive contamination information available.[7]

References
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0199". National Institute for Occupational Safety and Health (NIOSH).
- ^ COMMISSION DECISION of 20 September 2007 concerning the non-inclusion of 1,3-dichloropropene in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing that substance, Official Journal of the European Union, 25 September 2007.
- ^ Ludger Krähling, Jürgen Krey, Gerald Jakobson, Johann Grolig, Leopold Miksche "Allyl Compounds" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim, 2005. Published online: 15 June 2000. doi:10.1002/14356007.a01_425
- ^ Gerrit J. Poelarends, Christian P. Whitman "Evolution of enzymatic activity in the tautomerase superfamily: mechanistic and structural studies of the 1,3-dichloropropene catabolic enzymes" Bioorganic Chemistry 2004, Volume 32, Pages 376–392 doi:10.1016/j.bioorg.2004.05.006.
- ^ Robert L. Metcalf "Insect Control" in Ullmann’s Encyclopedia of Industrial Chemistry" Wiley-VCH, Wienheim, 2002. doi:10.1002/14356007.a14_263
- ^ a b [1]
- ^ [2]