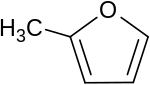
2-Methylfuran
| |

| |
| Names | |
|---|---|
| IUPAC name
2-Methylfuran
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.814 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H6O | |
| Molar mass | 82.10 g/mol |
| Appearance | Colorless to pale yellow/green liquid |
| Density | 0.927 g/mL |
| Boiling point | 63 to 66 °C (145 to 151 °F; 336 to 339 K) |
| 3000 mg/L (20 °C) | |
| Solubility in ethanol | Soluble |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Very flammable, harmful |
| NFPA 704 (fire diamond) | |
| Flash point | −22 °C; −8 °F; 251 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Methylfuran is a flammable, water-insoluble liquid[1] with a chocolate odor, found naturally in Myrtle and Dutch Lavender[2] used as a FEMA GRAS flavoring substance,[3] with the potential for use in alternative fuels.
Manufacture
2-Methylfuran is an article of commerce (chemical intermediate) and is normally manufactured by catalytic hydrogenolyis of furfural alcohol or via a hydrogenation-hydrogenolysis sequence from furfural in the vapor phase.[4]
See also
References
- ^ Kenneth Barbalace. "Chemical Database - 2-Methylfuran. EnvironmentalChemistry.com. 1995 - 2008. Accessed on-line: 8/26/2008". Retrieved 2008-08-26.
- ^ Jim Duke. "Dr. Duke's Phytochemical and Ethnobotanical Databases. [Online Database] 26 August 2008. 2-METHYL-FURAN". Retrieved 2008-08-26.
- ^ "2-methyl furan". The Good Scents Company. Retrieved 2008-08-26.
- ^ L. W. Burnette, et al., "Production of 2-Methylfuran by Vapor Phase Hydrogenation of Furfural" Industrial and engineering Chemistry, V40, P502-505 (1948).

