Islatravir
Appearance
| |
| Names | |
|---|---|
| IUPAC name
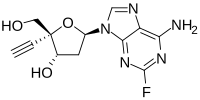
2'-Deoxy-4'-ethynyl-2-fluoroadenosine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H12FN5O3 | |
| Molar mass | 293.258 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) is a flavor enhancer for fermented soy sauce produced by Yamasa Corporation.
It also has activity against HIV in animal models.[1]
Biological activity
EFdA is a nucleoside analogue reverse transcriptase inhibitor that unlike other such inhibitors, inhibits HIV through multiple mechanisms.[1] providing rapid suppression of the virus, when tested in macaques and mice.[2] Nevertheless, there are HIV strains resistant to EFdA and research is ongoing.[3]
Refs
- ^ a b Michailidis, Eleftherios; Huber, Andrew D.; Ryan, Emily M.; Ong, Yee T.; Leslie, Maxwell D.; Matzek, Kayla B.; Singh, Kamalendra; Marchand, Bruno; Hagedorn, Ariel N.; Kirby, Karen A.; Rohan, Lisa C.; Kodama, Eiichi N.; Mitsuya, Hiroaki; Parniak, Michael A.; Sarafianos, Stefan G. (2014). "4'-Ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) Inhibits HIV-1 Reverse Transcriptase with Multiple Mechanisms". Journal of Biological Chemistry. 289 (35): 24533–48. doi:10.1074/jbc.M114.562694. PMC 4148878. PMID 24970894.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Stoddart, Cheryl A.; Galkina, Sofiya A.; Joshi, Pheroze; Kosikova, Galina; Moreno, Mary E.; Rivera, Jose M.; Sloan, Barbara; Reeve, Aaron B.; Sarafianos, Stefan G.; Murphey-Corb, Michael; Parniak, Michael A. (2015). "Oral Administration of the Nucleoside EFdA (4′-Ethynyl-2-Fluoro-2′-Deoxyadenosine) Provides Rapid Suppression of HIV Viremia in Humanized Mice and Favorable Pharmacokinetic Properties in Mice and the Rhesus Macaque". Antimicrobial Agents and Chemotherapy. 59 (7): 4190–8. doi:10.1128/AAC.05036-14. PMC 4468726. PMID 25941222.
- ^ Bruno Marchand. "The Crystal Structure of EFdA‐Resistant HIV‐1 Reverse Transcriptase Reveals Structural Changes in the Polymerase Active Site" (PDF).
