4-Nitroaniline

| |
| Names | |
|---|---|
| Other names
4-nitroaniline
1-amino-4-nitrobenzene p-nitrophenylamine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.555 |
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H6N2O2 | |
| Molar mass | 138.12 g/mol |
| Appearance | yellow or brown powder |
| Odor | faint, ammonia-like |
| Density | 1.437 g/ml, solid |
| Melting point | 146-149 °C(lit.) |
| Boiling point | 332 °C |
| 0.8 mg/ml at 18.5°C (IPCS) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic |
| NFPA 704 (fire diamond) | |
| Flash point | 199 °C (390 °F; 472 K) |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
4-Nitroaniline, p-nitroaniline or 1-amino-4-nitrobenzene is an organic compound with the formula C6H6N2O2. It is an organic chemical compound, consisting of a phenyl group attached to an amino group which is para to a nitro group. This chemical is commonly used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals and gasoline, in gum inhibitors, poultry medicines, and as a corrosion inhibitor.
Synthesis
It is produced industrially via the amination of 4-nitrochlorobenzene:[1]
- ClC6H4NO2 + 2 NH3 → H2NC6H4NO2 + NH4Cl
Below is a laboratory synthesis of p-nitroaniline from aniline. The key step in this reaction sequence is an electrophilic aromatic substitution to install the nitro group para to the amino group. After this reaction, a separation must be performed to remove 2-nitroaniline, which is also formed in a small amount during the reaction.[2]
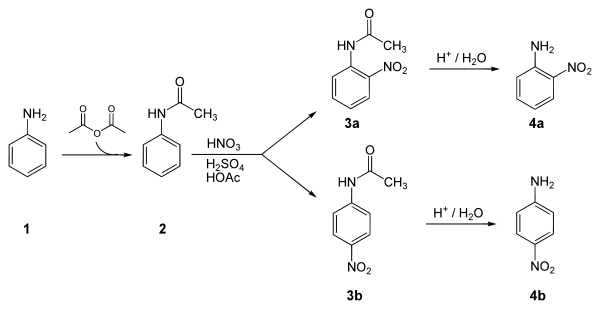
Applications
4-Nitroaniline is mainly consumed industrially as a precursor to p-phenylenediamine, an important dye. The reduction is effected using iron metal and by catalytic hydrogenation.[1]
It is a starting material for the synthesis of Para Red, the first Azo dye:[3]

Toxicity
The compound is toxic by way of inhalation, ingestion, and absorption, and should be handled with care. Its LD50 in rats is 750 mg/kg when administered orally. p-Nitroaniline is particularly harmful to all aquatic organisms, and can cause long-term damage to the environment if released as a pollutant.
References
- ^ a b Gerald Booth "Nitro Compounds, Aromatic in Ullmann's Encyclopedia of Industrial Chemistry, 7th Ed.; Wiley-VCH: Weinheim, 2005. doi:10.1002/14356007.a17_411
- ^ Mohrig, J.R.; Morrill, T.C.; Hammond, C.N.; Neckers, D.C. "Synthesis 5: Synthesis of the Dye Para Red from Aniline." Experimental Organic Chemistry. Freeman: New York, NY, 1997; pp 456-467.
- ^ Williamson, Kenneth L. (2002). Macroscale and Microscale Organic Experiments, Fourth Edition. Houghton-Mifflin. ISBN 0-618-19702-8.
External links
- Safety (MSDS)data for p-nitroaniline
- MSDS Sheet for p-nitroaniline
- Sigma-Aldrich Catalog data
- CDC - NIOSH Pocket Guide to Chemical Hazards

