Benzylideneacetone

| |

| |
| Names | |
|---|---|
| IUPAC name
(E)-4-Phenylbut-3-ene-2-one
| |
| Other names
Benzalacetone
Benzylideneacetone Methyl styryl ketone Benzylidene acetone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.015.989 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H10O | |
| Molar mass | 146.19 g/mol |
| Appearance | pale yellow solid |
| Density | 1.008 g/cm3 |
| Melting point | 39 to 42 °C (102 to 108 °F; 312 to 315 K) |
| Boiling point | 260 to 262 °C (500 to 504 °F; 533 to 535 K) |
| 1.3 g/L | |
| Solubility in other solvents | nonpolar solvents |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
irritant |
| GHS labelling: | |

| |
| Warning | |
| H315, H317, H319, H335 | |
| P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P333+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
| Flash point | 116 °C (241 °F; 389 K) |
| Related compounds | |
Related compounds
|
Dibenzylideneacetone cinnamaldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzylideneacetone is the organic compound described by the formula C6H5CH=CHC(O)CH3. Although both cis- and trans-isomers are possible for the α,β-unsaturated ketone, only the trans isomer is observed. Its original preparation demonstrated the scope of condensation reactions to construct new, complex organic compounds.[1] Benzylideneacetone is used as a flavouring ingredient in food and perfumes.[2]
Preparation
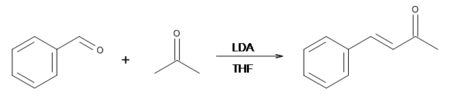
Benzylideneacetone can be efficiently prepared by the NaOH-induced condensation of the readily available reagents acetone and benzaldehyde:[3]
However, the benzylideneacetone formed via this reaction can undergo another Claisen-Schmidt condensation with another molecule of benzaldehyde to form dibenzylideneacetone. Because relatively weak bases such as NaOH make very little of the enolate ion at equilibrium, there is still a lot of unreacted base left in the reaction mixture, which can go on and remove protons from the alpha carbon of benzylideneacetone, allowing it to undergo another Claisen-Schmidt condensation and make dibenzylideneacetone.[4]
If, on the other hand, lithium diisopropylamide (LDA) is used as the base, all of the acetone will deprotonated, making enolate ion quantitatively. Therefore, the most efficient way to make benzylideneacetone is to use equimolar amounts of LDA (in THF), acetone, and benzaldehyde. The reaction must be done with anhydrous chemicals as LDA breaks down in water.[5]
Reactions
As with most methyl ketones, benzylideneacetone is moderately acidic at the alpha position, and it can be readily deprotonated to form the corresponding enolate[6]

The compound undergoes the reactions expected for its collection of functional groups: e.g., the double bond adds bromine, the heterodiene adds electron-rich alkenes in Diels-Alder reactions to give dihydropyrans, the methyl group undergoes further condensation with benzaldehyde to give dibenzylideneacetone, and the carbonyl forms hydrazones. It reacts with Fe2(CO)9 to give (benzylideneacetone)Fe(CO)3, a reagent for transferring the Fe(CO)3 unit to other organic substrates.[7]
- Hydrogenation of benzylideneacetone results in a preparation of benzylacetone.
- The reaction of 4-Hydroxycoumarin with this compound yields Warfarin.
References
- ^ Claisen, L. "Über die Einwirkung von Aceton auf Furfural und auf Benzaldehyd bei Gegenwart von Alkalilauge" Berichte der deutschen chemischen Gesellschaft 1881, volume 14, p 2468-2471.
- ^ Opdyke, D. L. J. (2013). Monographs on Fragrance Raw Materials: A Collection of Monographs Originally Appearing in Food and Cosmetics Toxicology. Elsevier. p. 135. ISBN 9781483147970.
- ^ Drake, N. L.; Allen, Jr. P. "Benzalacetone". Organic Syntheses; Collected Volumes, vol. 1, p. 77.
- ^ Moya-Barrios, R. CHEM 2402 Lab Manual, Winter 2016. Dalhousie University, Department of Chemistry
- ^ Bruice, Paula Yurkanis. Organic Chemistry, 7th Edition. Pearson Education, 2014. ISBN 0-321-80322-1
- ^ Danheiser, R. L.; Miller, R. F.; Brisbois, R. G. (1990). "Detrifluoroacetylative Diazo Group Transfer: (E)-1-Diazo-4-phenyl-3-buten-2-one". Organic Syntheses. 73: 134; Collected Volumes, vol. 9, p. 197.
- ^ Knölker, H.-J. "(η4-Benzylideneacetone)tricarbonyliron" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. Onlinedoi:10.1002/047084289X.rb058.