Contrast CT

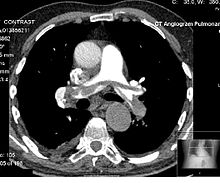
Contrast CT, or contrast enhanced computed tomography (CECT), is X-ray computed tomography (CT) using radiocontrast. Radiocontrasts for X-ray CT are generally iodine-based types.[1] This is useful to highlight structures such as blood vessels that otherwise would be difficult to delineate from their surroundings. Using contrast material can also help to obtain functional information about tissues. Often, images are taken both with and without radiocontrast. CT images are called precontrast or native-phase images before any radiocontrast has been administrated, and postcontrast after radiocontrast administration.[2]
Bolus tracking

Bolus tracking is a technique to optimize timing of the imaging. A small bolus of radio-opaque contrast media is injected into a patient via a peripheral intravenous cannula. Depending on the vessel being imaged, the volume of contrast is tracked using a region of interest (abbreviated "R.O.I.") at a certain level and then followed by the CT scanner once it reaches this level. Images are acquired at a rate as fast as the contrast moving through the blood vessels.
This method of imaging is used primarily to produce images of arteries, such as the aorta, pulmonary artery, cerebral, carotid and hepatic arteries.
Washout
"Washout" is where tissue loads radiocontrast during arterial phase, but then returns to a rather hypodense state in venous or later phases. This is a property of for example hepatocellular carcinoma as compared to the rest of the liver parenchyma.[3]
Phases
Depending on the purpose of the investigation, there are standardized protocols for time intervals between intravenous radiocontrast administration and image acquisition, in order to visualize the dynamics of contrast enhancements in different organs and tissues.[4] The main phases thereof are as follows:[5]
| Phase | Time from injection[5] | Time from bolus tracking[5] | Targeted structures and findings[5] |
|---|---|---|---|
| Non-enhanced CT (NECT) | - | - |
|
| Pulmonary arterial phase | 6-13 sec[6] | - |
|
| Pulmonary venous phase | 17-24 sec[6] | - | |
| Early systemic arterial phase | 15-20 sec | immediately |
|
| Late systemicarterial phase Sometimes also called "arterial phase" or "early venous portal phase" |
35-40 sec | 15-20 sec |
|
| Pancreatic phase | 30[8] or 40[9] - 50[9] sec | 20-30 sec |
|
| Hepatic (most accurate) or late portal phase | 70-80 sec | 50-60 sec |
|
| Nephrogenic phase | 100 sec | 80 sec |
|
| Systemic venous phase | 180 sec[citation needed] | 160 sec |
|
| Delayed phase Sometimes called "wash out phase" or "equilibrium phase" |
6[5]-15[citation needed] minutes | 6[5]-15[citation needed] minutes |
|
Angiography
CT angiography is a contrast CT taken at the location and corresponding phase of the blood vessels of interest, in order to detect vascular diseases. For example, an abdominal aortic angiography is taken in the arterial phase in the abdominal level, and is useful to detect for example aortic dissection.[10]
Amount

Adults
The following table shows the preferable volume in normal weight adults. However, dosages may need to be adjusted or even withheld in patients with risks of iodinated contrast, such as hypersensitivity reactions, contrast-induced nephropathy, effects on thyroid function or adverse drug interactions.
| Exam | Iodine concentration | Comments | |||
|---|---|---|---|---|---|
| 300 mg/ml | 350 mg/ml | 370 mg/ml | |||
| CT of brain | 95ml[11] | 80 ml[11] | 75 ml[11] | ||
| CT of thorax | Overall | 70 - 95 ml[notes 1] | 60 - 80 ml[notes 1] | 55 - 75 ml[notes 1] | Parenchymal changes of the lung can often be evaluated adequately without the use of intravenous contrast. |
| CT pulmonary angiogram | 20 ml[notes 2] | 17 ml[notes 2] | 15 ml[notes 2] | Minimal amount when using specific low-contrast protocol.[notes 2] | |
| CT of abdomen | Overall | 70 ml[11] | 60 ml[11] | 55 ml[11] | |
| Liver | 55 ml[notes 3] | 45 ml[notes 3] | 40-45 ml[notes 3] | Minimal required amount.[notes 3] | |
| CT angiography | 25 ml[notes 4] | 20 ml[notes 4] | When using specific low-contrast protocol.[notes 4] | ||
The dose should be adjusted in those not having normal body weight, and in such cases the adjustment should be proportional to the lean body mass of the person. In obese patients, the Boer formula is the method of choice (at least in those with body mass index (BMI) between 35 and 40):[12]
For men: Lean body mass = (0.407 × W) + (0.267 × H) − 19.2
For women: Lean body mass = (0.252 × W) + (0.473 × H) − 48.3
Children
Standard doses in children:[13]
| Exam | Concentration of iodine | |
|---|---|---|
| 300 mg/ml | 350 mg/ml | |
| Generally | 2.0 ml/kg | 1.7 ml/kg |
| CT of brain, neck or thorax | 1.5 ml/kg | 1.3 ml/kg |
Adverse effects
Iodinated contrast agents may cause allergic reactions, contrast-induced nephropathy, hyperthyroidism and possibly metformin accumulation. However, there are no absolute contraindications to iodinated contrast, so the benefits needs to be weighted against the risks.[14]
As with CT scans in general, the radiation dose can potentially increase the risk of radiation-induced cancer.
The injection of iodinated contrast agents may sometimes lead to its extravasation[15]
See also
Notes
- ^ a b c 0.3–0.4 gI/kg in a 70kg individual, according to:
- Iezzi, Roberto; Larici, Anna Rita; Franchi, Paola; Marano, Riccardo; Magarelli, Nicola; Posa, Alessandro; Merlino, Biagio; Manfredi, Riccardo; Colosimo, Cesare (2017). "Tailoring protocols for chest CT applications: when and how?". Diagnostic and Interventional Radiology. 23 (6): 420–427. doi:10.5152/dir.2017.16615. ISSN 1305-3825. PMC 5669541. PMID 29097345.
- ^ a b c d Using dual energy CTA (such as 90/150SnkVp), according to:
- Leroyer, Christophe; Meier, Andreas; Higashigaito, Kai; Martini, Katharina; Wurnig, Moritz; Seifert, Burkhardt; Keller, Dagmar; Frauenfelder, Thomas; Alkadhi, Hatem (2016). "Dual Energy CT Pulmonary Angiography with 6g Iodine—A Propensity Score-Matched Study". PLOS ONE. 11 (12): e0167214. Bibcode:2016PLoSO..1167214M. doi:10.1371/journal.pone.0167214. ISSN 1932-6203. PMC 5132396. PMID 27907049.
- ^ a b c d The liver generally needs an enhancement of at least 30 HU for proper evaluation according to:
- Multislice CT (3 ed.). Springer-Verlag Berlin and Heidelberg GmbH & Co. KG. 2010. ISBN 9783642069680.
- Bae, Kyongtae T. (2010). "Intravenous Contrast Medium Administration and Scan Timing at CT: Considerations and Approaches". Radiology. 256 (1): 32–61. doi:10.1148/radiol.10090908. ISSN 0033-8419. PMID 20574084.
- ^ a b c CT-angiography in a 70kg person, with 100-150 mg I/kg by using 80 kVp, mAs-compensation for constant CNR, fixed injection duration adapted to scan time, automatic bolus tracking and a saline chaser, according to:
- Nyman, Ulf (2012). "Contrast Medium-Induced Nephropathy (CIN) Gram-Iodine/GFR Ratio to Predict CIN and Strategies to Reduce Contrast Medium Doses". Coronary Interventions. doi:10.5772/29992. ISBN 978-953-51-0498-8.
References
- ^ Webb, W. Richard; Brant, Wiliam E.; Major, Nancy M. (2014). Fundamentals of Body CT. Elsevier Health Sciences. p. 152. ISBN 9780323263580.
- ^ Dahlman P, Semenas E, Brekkan E, Bergman A, Magnusson A (2000). "Detection and Characterisation of Renal Lesions by Multiphasic Helical Ct". Acta Radiologica. 41 (4): 361–366. doi:10.1080/028418500127345479. PMID 10937759. S2CID 27758886.
- ^ Choi, Jin-Young; Lee, Jeong-Min; Sirlin, Claude B. (2014). "CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma: Part II. Extracellular Agents, Hepatobiliary Agents, and Ancillary Imaging Features". Radiology. 273 (1): 30–50. doi:10.1148/radiol.14132362. ISSN 0033-8419. PMC 4263770. PMID 25247563.
- ^ Bae, Kyongtae T. (2010). "Intravenous Contrast Medium Administration and Scan Timing at CT: Considerations and Approaches". Radiology. 256 (1): 32–61. doi:10.1148/radiol.10090908. ISSN 0033-8419. PMID 20574084.
- ^ a b c d e f Robin Smithuis. "CT contrast injection and protocols". Radiology Assistant. Retrieved 2017-12-13.
- ^ a b Page 584 in: Ákos Jobbágy (2012). 5th European Conference of the International Federation for Medical and Biological Engineering 14 - 18 September 2011, Budapest, Hungary. Volume 37 of IFMBE Proceedings. Springer Science & Business Media. ISBN 9783642235085.
- ^ Pavan Nandra (2018). "Introducing the use of Flash CTPA; how does it compare to standard CTPA?". Postering. doi:10.1594/ecr2018/C-1831.
- ^ Raman SP, Fishman EK (2012). "Advances in CT Imaging of GI Malignancies". Gastrointest Cancer Res. 5 (3 Suppl 1): S4-9. PMC 3413036. PMID 22876336.
- ^ a b c Otto van Delden and Robin Smithuis. "Pancreas - Carcinoma". Radiology Assistant. Retrieved 2017-12-15.
- ^ Page 424 in: Stuart E. Mirvis, Jorge A. Soto, Kathirkamanathan Shanmuganathan, Joseph Yu, Wayne S. Kubal (2014). Problem Solving in Emergency Radiology E-Book. Elsevier Health Sciences. ISBN 9781455758395.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f "New Zealand Datasheet" (PDF). New Zealand Medicines and Medical Devices Safety Authority. Retrieved 2018-10-16.
- ^ Caruso, Damiano; De Santis, Domenico; Rivosecchi, Flaminia; Zerunian, Marta; Panvini, Nicola; Montesano, Marta; Biondi, Tommaso; Bellini, Davide; Rengo, Marco; Laghi, Andrea (2018). "Lean Body Weight-Tailored Iodinated Contrast Injection in Obese Patient: Boer versus James Formula". BioMed Research International. 2018: 1–6. doi:10.1155/2018/8521893. ISSN 2314-6133. PMC 6110034. PMID 30186869.
- ^ Nievelstein, Rutger A. J.; van Dam, Ingrid M.; van der Molen, Aart J. (2010). "Multidetector CT in children: current concepts and dose reduction strategies". Pediatric Radiology. 40 (8): 1324–1344. doi:10.1007/s00247-010-1714-7. ISSN 0301-0449. PMC 2895901. PMID 20535463.
- ^ Stacy Goergen. "Iodine-containing contrast medium". InsideRadiology - The Royal Australian and New Zealand College of Radiologists. Retrieved 2019-02-22. Page last modified on 26/7/2017
- ^ Hrycyk J, Heverhagen JT, Böhm I (2019). "What you should know about prophylaxis and treatment of radiographic and magnetic resonance contrast medium extravasation". Acta Radiol. 60 (4): 496–500. doi:10.1177/0284185118782000. PMID 29896979. S2CID 48360725.
External links
- "CT with IV contrast in low renal function". Radlines.org.
