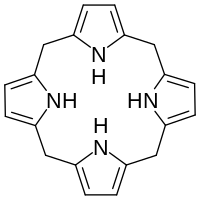
Hexahydroporphine
| |
| Names | |
|---|---|
| IUPAC name
5,10,15,20,22,24-Hexahydroporphyrin
| |
| Other names
Porphyrinogen; Calix[4]pyrrole
| |
| Identifiers | |
3D model (JSmol)
|
|
| 7545460 | |
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H20N4 | |
| Molar mass | 316.408 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 185 °C (365 °F; 458 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hexahydroporphine is an organic chemical compound with formula C20H20N4. The molecule consists of four pyrrole rings connected by methylene bridges −CH2− into a larger (non-aromatic) macrocycle ring, which makes it one of the simplest tetrapyrroles, and the simplest "true" one. As indicated by the name, it may be viewed as derived from porphine by the addition of six hydrogen atoms: four on the methine bridges, and two on the nitrogen atoms.
Hexahydroporphine does not occur in nature, but is the core of porphyrinogens such as uroporphyrinogen III (UROGEN), which are precursors of many porphyrins — derivatives of porphine of great biological importance. The six hydrogens of that core are removed at a later metabolic stage by the enzyme protoporphyrinogen oxidase. Because of this connection, the compound is also called (unsubstituted) porphyrinogen.
The compound is a colorless solid, soluble in dichloromethane, acetone, and diethyl ether. It decomposes at 185°C.[1]
Preparation
[edit]Derivatives of hexahydroporphine, with various groups attached to the pyrrole or methylene bridges, occur in nature and have been studied for a long time.[2][3][4][5] The unsubstituted compound, however, has been synthesized in good yield only in 2001. It can be obtained by successive condensations of 2,5-bis(hydroxymethyl)pyrrole and pyrrole, with a tripyrrole intermediate.[1]
The compound can also be prepared by reduction of porphin-zinc complexes.[6][7]
See also
[edit]References
[edit]- ^ a b Shozo Taniguchi, Hikaru Hasegawa, Shoko Yanagiya, Yusuke Tabeta, Yoshiharu Nakano, and Masahiko Takahashi (2001): "The first isolation of unsubstituted porphyrinogen and unsubstituted 21-oxaporphyrinogen by the ‘3+1’ approach from 2,5-bis(hydroxymethyl)pyrrole and tripyrrane derivatives". Tetrahedron, volume 57, issue 11, pages 2103-2108. doi:10.1016/S0040-4020(01)00059-X
- ^ P. S. Clezy and C. J. R. Fookes (1977): "The chemistry of pyrrolic compounds. XXXVIII. The synthesis of hexahydroporphyrin a and related compounds". Australian Journal of Chemistry, volume 30, issue 8, pages 1799–1813. doi:10.1071/CH9771799
- ^ A. H. Jackson (2009): "The total synthesis of pyrrole pigments". In John ApSimon (ed.), The Total Synthesis of Natural Products, volume 1; Wiley, 624 pages.ISBN 9780470129500
- ^ Goutam K. Lahiri and Alan M. Stolzenberg(1993): "Facile Formation of Hexahydroporphyrin Complexes by Reduction of Octaethylisobacteriochlorinnickel(II)". Angewandte Chemie, volume 32, issue 3, pages 429-432. doi:10.1002/anie.199304291
- ^ Hidemitsu Uno, Takashi Inoue, Yumiko Fumoto, Motoo Shiro, and Noboru Onomeso (2000): "Unsubstituted Porphyrinogens and Hexaphyrinogens: The First X-ray Characterization" Journal of the American Chemical Society, volume 122, issue 28, pages 6773–6774. doi:10.1021/ja000482e
- ^ Gilbert R. Seely and Melvin Calvin (1955): "Photochemical Studies of the Porphyrins. III. Photoreduction of a Porphyrin by Benzoin" Journal of Chemical Physics, volume 23, issue 6, pages 1068–. doi:10.1063/1.1742192
- ^ Gilbert R. Seely (1957): "Molecular Orbital Study of the Porphyrins". Journal of Chemical Physics, volume 27, issue 1, pages 125–. doi:10.1063/1.1743651
