POTEB
| POTEB | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | POTEB, A26B1, CT104.5, POTE-15, POTE15, POTE ankyrin domain family member B, POTEB2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 608912; HomoloGene: 136720; GeneCards: POTEB; OMA:POTEB - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
POTE ankyrin domain family, member B is a protein in humans that is encoded by the POTEB gene.[3](Prostate, Ovary, Testes Expressed ankyrin domain family member B).It is most likely involved in mediating protein-protein interaction via its 5 ankyrin domains.[4] POTEB is most probably aids in intracellular signaling, but is not likely to be a secreted or nuclear protein.[4] POTEB's function is likely to be regulated via 17 potential phosphorylation sites.[5] There is currently no evidence to suggest that POTEB has nuclear localization signals.[6]
Gene
POTEB is located at 15q11.2 on chromosome 15 in humans and is transcribed from the reverse DNA strand. POTEB is also known as POTEB3 and POTE15.[7] The POTEB gene is 47,547 base pairs in length and is composed of 11 exons.[7]

mRNA
The POTEB gene can be transcribed to create four potential mRNAs. However, only one of these mRNAs, possessing all 11 exons, is capable of being translated to the POTEB protein.[8] The three other transcripts do not encode proteins.

Protein
The POTEB protein is composed of 544 amino acids and, according to bioinformatic analyses, has a molecular weight of 61.7 kDa. It has an isoelectric point of 5.68.[9] Its most common amino acids are leucine and glutamic acid, which account for 11% and 10.3% of the protein respectively.[9] However, this is normal for human proteins. POTEB is most likely a cytoplasmic protein[10] that is phosphorylated at 17 serines, threonines, and tyrosines located throughout the length of the protein,[5] but concentrated at the C-terminus of the protein. Its secondary structure is mainly five helical ankyrin repeat domains, which contain the TALHL motif. There is also one myristoylation site on the protein, close to the N-terminus.[11]

Expression
POTEB is expressed at high levels in the human prostate, ovary, and testes. However, there is also evidence to show that it is expressed at low levels in embryonic stem cells, the nasopharyngeal region, and in breast tissue.[12][13] In embryonic stem cells, differentiation is likely to turn off the expression of POTEB while in breast cancer, triple negative cells are found to have no POTEB expression suggesting a role in cancer-activated pathways.[13]

Some studies have used POTEB probes to study the expression of POTEB in the human brain. However, the only region with notable POTEB expression is the cerebellar cortex, responsible for motor function and some cognitive functions.[14]

Regulation of Expression
POTEB expression is likely regulated by E-box binding factors and Krueppel-like transcription factors, along with nuclear factor kappa B (NF-κB) transcription factors.[15]
POTEB expression could be regulated by the binding of transcription factors to intron 1 of the pre-mRNA, leading to the production of a truncated mRNA which is not translated. Alternatively, POTEB expression could be downregulated by the formation of stem loops close to the start codon.[16] There are no known ubiquitination sites in POTEB that could aid in regulating POTEB function and stability.

Function
POTEB is most likely involved in mediating protein-protein interaction via its 5 ankyrin domains.[4] POTEB is most probably aids in intracellular signaling, but is not likely to be a secreted or nuclear protein[4] as it is unlikely to contain nuclear localization signals.[6] POTEB's function is likely to be regulated via 17 potential phosphorylation sites[5] which determine how the ankyrin domains interact with other proteins.[17]
Protein-Protein Interactions
There have been no studies published confirming the interaction of POTEB with other human proteins. However, there is unpublished data suggesting an interaction between POTEB and alpha-1-B glycoproteins, APOBEC1 complementation factors, and alpha-2-macroglobulin.[18] This data is based on affinity capture- mass spectrometry.
Clinical Significance
POTEB expression is low or completely reduced in triple-negative breast cancer cells when compared to other types of breast cancer cells.[13] This suggests POTEB’s involvement in intracellular signaling pathways that suppress cancer, or in pathways that regulate the normal growth and division of cells.
Homology
Paralogs
POTEB has 8 predicted paralogs (According to protein sequence) in humans, with most paralogs being located on different human chromosomes.[19][20] It is speculated that this large number of paralogs arose from multiple duplication events.[10]
| Paralog name | Accession number | % Identity | Amino acids | Chromosome # |
|---|---|---|---|---|
| POTE 15B | AAS58869.1 | 99 | 544 | Chromosome 15 |
| POTE C | NP_001131143.1 | 90 | 542 | Chromosome 18 |
| POTE H | NP_001129685.1 | 89 | 545 | Chromosome 22 |
| POTE J | NP_001264012.1 | 86 | 1038 | Chromosome 2 |
| POTE 14A | AAS58868.1 | 82 | 508 | Chromosome 14 |
| POTE G | NP_001005356.1 | 82 | 508 | Chromosome 14 |
| POTE E | XP_016859648.1 | 80 | 967 | Chromosome 2 |
| POTE I | NP_001264335.1 | 80 | 1075 | Chromosome 2 |
Orthologs
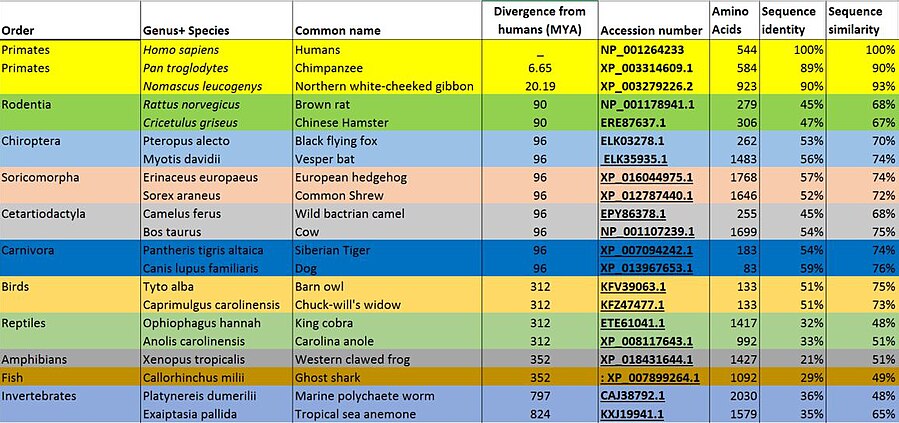
POTEB orthologs have been found in mammals, birds, reptiles, amphibians, fish, and even in invertebrates such as sea anemones and marine polychaete worms.[19] These orthologs share a similarity with POTEB largely due to the presence of ankyrin repeats, suggesting that ankyrin domain-containing proteins have been conserved over millions of years. POTEB orthologs have not been found in plants, unicellular eukaryotes, bacteria and archaea.[19]
References
- ^ a b c ENSG00000288347 GRCh38: Ensembl release 89: ENSG00000233917, ENSG00000288347 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Entrez Gene: POTE ankyrin domain family, member B". Retrieved 2012-11-26.
- ^ a b c d Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY (June 2004). "The ankyrin repeat as molecular architecture for protein recognition". Protein Science. 13 (6): 1435–48. doi:10.1110/ps.03554604. PMC 2279977. PMID 15152081.
- ^ a b c "NetPhos 3.1 Server". www.cbs.dtu.dk. Retrieved 2017-05-06.
- ^ a b "SignalP 4.1 Server". www.cbs.dtu.dk. Retrieved 2017-05-06.
- ^ a b Database, GeneCards Human Gene. "POTEB Gene - GeneCards | POTEB Protein | POTEB Antibody". www.genecards.org. Retrieved 2017-05-06.
- ^ "Chromosome 15: 21,846,329-21,877,703 - Region in detail - Homo sapiens - Ensembl genome browser 88". useast.ensembl.org. Retrieved 2017-05-06.
- ^ a b Workbench, NCSA Biology. "SDSC Biology Workbench". workbench.sdsc.edu. Retrieved 2017-05-06.
- ^ a b Hahn Y, Bera TK, Pastan IH, Lee B (February 2006). "Duplication and extensive remodeling shaped POTE family genes encoding proteins containing ankyrin repeat and coiled coil domains". Gene. 366 (2): 238–45. doi:10.1016/j.gene.2005.07.045. PMID 16364570.
- ^ "ExPASy - Myristoylation tool". web.expasy.org. Retrieved 2017-05-07.
- ^ Bera TK, Saint Fleur A, Ha D, Yamada M, Lee Y, Lee B, Hahn Y, Kaufman DS, Pera M, Pastan I (April 2008). "Selective POTE paralogs on chromosome 2 are expressed in human embryonic stem cells". Stem Cells and Development. 17 (2): 325–32. doi:10.1089/scd.2007.0079. PMC 7233169. PMID 18447647.
- ^ a b c "GEO Accession viewer". www.ncbi.nlm.nih.gov. Retrieved 2017-05-06.
- ^ Wolf U, Rapoport MJ, Schweizer TA (2009-07-01). "Evaluating the affective component of the cerebellar cognitive affective syndrome". The Journal of Neuropsychiatry and Clinical Neurosciences. 21 (3): 245–53. doi:10.1176/jnp.2009.21.3.245. PMID 19776302.
- ^ "Genomatix - NGS Data Analysis & Personalized Medicine". www.genomatix.de. Retrieved 2017-05-06.
- ^ "UNAFold | mfold.rit.albany.edu". unafold.rna.albany.edu. Retrieved 2017-05-06.
- ^ Nelson WJ, Veshnock PJ (1987-08-06). "Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells". Nature. 328 (6130): 533–6. Bibcode:1987Natur.328..533N. doi:10.1038/328533a0. PMID 3039371.
- ^ Mike Tyers Lab. "The BioPlex Network of Human Protein Interactions: Additional Unpublished AP-MS Results | BioGRID". thebiogrid.org. Retrieved 2017-05-07.
- ^ a b c "Protein BLAST: search protein databases using a protein query". blast.ncbi.nlm.nih.gov. Retrieved 2017-05-07.
- ^ Bera TK, Huynh N, Maeda H, Sathyanarayana BK, Lee B, Pastan I (August 2004). "Five POTE paralogs and their splice variants are expressed in human prostate and encode proteins of different lengths". Gene. 337: 45–53. doi:10.1016/j.gene.2004.05.009. PMID 15276201.
- ^ "TimeTree :: The Timescale of Life". www.timetree.org. Retrieved 2017-05-07.


