Tetrakis(trimethylsilyl)silane
Appearance
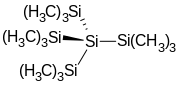
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,1,3,3,3-Hexamethyl-2,2-bis(trimethylsilyl)trisilane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.156.064 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H36Si5 | |
| Molar mass | 320.845 g·mol−1 |
| Appearance | colorless solid |
| Density | 0.806 g/cm3 |
| Melting point | 319–321 °C (606–610 °F; 592–594 K) sealed tube |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetrakis(trimethylsilyl)silane is the organosilicon compound with the formula (Me3Si)4Si (where Me = CH3). It is a colorless sublimable solid with a high melting point. The molecule has tetrahedral symmetry. The compound is notable as having silicon bonded to four other silicon atoms, like in elemental silicon.
Preparation and reactions
[edit]The compound is prepared by the reaction of trimethylsilyl chloride, silicon tetrachloride, and lithium:[1][2]
- 4 Me3SiCl + SiCl4 + 8 Li → (Me3Si)4Si + 8 LiCl
The compound is a precursor to tris(trimethylsilyl)silyl lithium by reaction with methyl lithium:[2]
- (Me3Si)4Si + MeLi → (Me3Si)3SiLi + Me4Si
The organolithium compound (Me3Si)3SiLi is a versatile reagent, e.g. to tris(trimethylsilyl)silane ((Me3Si)3SiH).
See also
[edit]References
[edit]- ^ Gilman, H.; Smith, C. L. (1967). "Tetrakis(trimethylsilyl)silane". Journal of Organometallic Chemistry. 8 (2): 245–253. doi:10.1016/S0022-328X(00)91037-4.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Joachim Dickhaut, Bernd Giese (1992). "Tris(trimethylsilyl)silane". Org. Synth. 70: 164. doi:10.15227/orgsyn.070.0164.
