Intermittent hypoxia
| Intermittent hypoxia | |
|---|---|
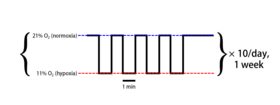 Example of a typical intermittent hypoxia protocol | |
| Other names | Episodic hypoxia |
Intermittent hypoxia (also known as episodic hypoxia) is an intervention in which a person or animal undergoes alternating periods of normoxia and hypoxia. Normoxia is defined as exposure to oxygen levels normally found in earth's atmosphere (~21% O2) and hypoxia as any oxygen levels lower than those of normoxia. Normally, exposure to hypoxia is negatively associated to physiological changes to the body, such as altitude sickness.[1] However, when used in moderation, intermittent hypoxia may be used clinically as a means to alleviate various pathological conditions.[2]
General Mechanisms

When used as a rehabilitative intervention, particularly for respiration and walking, intermittent hypoxia typically works by using long-term facilitation (LTF). LTF, which is synonymous to long-term potentiation, occurs when there are long-term increases in synaptic strength due to synaptic plasticity.[3] In the case of intermittent hypoxia, these increases in synaptic strength result in increased motor output.[4][5]
Reduced partial pressures of oxygen in the arteries due to intermittent hypoxia are sensed by and stimulate the carotid body, a chemoafferent receptor.[6][7] The activated carotid body triggers the release of serotonin that attach to serotonin receptors on the surface of motoneurons, such as the phrenic motoneuron in the case of respiratory recovery.[5] This signal transduction pathway then uses downstream molecules such as TrkB,[8] BDNF,[8] and PKA[9] to increase the synaptic output of the involved motor neuron which in turn increases the motor output of the involved muscles and, thus, decreases functional impairment. As the amount of intermittent hypoxia changes the amount of serotonin release and, as a result, the amount of LTF, this process exhibits metaplasticity.[10] Metaplasticity occurs when the LTF is itself plastic or variable.
Intermittent hypoxia-induced LTF has also been demonstrated in carotid denervated rats, suggesting that synaptic plasticity due to intermittent hypoxia also works through other mechanisms outside of carotid chemoafferents.[11]
Aside from this, intermittent hypoxia also alters overall nitric oxide production, concentration, and gene expression, which occurs due to cardiovascular adaptations to hypoxia.[12] This mechanism is relevant when used as a means to decrease hypertension[13] or increase bone mineral density[14]
Dosage
| Type | Example |
|---|---|
| Hypoxia severity | FiO2 of 0.10 |
| Episodic duration | 1 minute per episode |
| Episodes per day | 10 episodes/day |
| Pattern of presentation | Every other day |
| Cumulative exposure duration | 24 cumulative hours |
An understanding of proper dosage is needed in order to design an effective intermittent hypoxia protocol, particularly due to the comorbidities associated with hypoxia. For example, intermittent hypoxia has been shown to induce LTF in rats while continuous hypoxia does not.[15] And acute IH shows no evidence of the hippocampal cell death found in rats while chronic intermittent hypoxia exposure does[16]
Though intermittent hypoxia has been used for various therapeutic applications across a number of physiological system, there is a general consensus in what can be considered a safe and beneficial amount of intermittent hypoxia. Such a protocol would involve a fraction of inspired oxygen (FiO2) ranging between 0.09 – 0.16 with 3 – 15 episodes per day with comorbidities found in the range of a FiO2 of 0.03 – 0.08 and 48 – 2400 episodes per day.[2]
Pathological and beneficial effects
| Pathological effects[2] | Beneficial effects[2] |
|---|---|
| Systemic hypertension | Decrease arterial hypertension |
| Obesity | Weight loss |
| Insulin resistance | Increase glucose tolerance |
| Increase sympathetic activation | Strengthen immune response |
| Cognitive deficits | Enhance spatial learning and memory |
| Inflammation | Decrease inflammation |
Therapeutic applications
Though intermittent hypoxia is initially involved with only the respiratory system, its downstream effects allow it to also be used as an effective rehabilitative intervention in a number of different biological systems in both animals and humans.
LTF
For the respiratory system, the LTF facilitated by intermittent hypoxia aids in increasing phrenic motor nerve output. This has been shown to help people with obstructive sleep apnea[17] and COPD.[18] The ability to increase muscle activity, specifically for walking, has also been demonstrated in both rats[16] and humans[19] after spinal cord injury.
Hippocampal neurogenesis
Hippocampal neurogenesis has also been demonstrated in rats subjected to intermittent hypoxia. This neurogenesis has shown related cognitive improvements such as enhanced learning and memory[20][21] as well as overall increases in spatial cognitive ability.[22] Additionally, antidepressant-like effects are exhibited in rats undergoing such treatment.[23]
Nitric oxide production
Nitric oxide level changes due to intermittent hypoxia also provide potential benefits. People with hypertension have shown decreases in blood pressure.[13][24] Increases in bone mineral density in rats has also been attributed to this process.[14] Such changes to nitric oxide levels also aid in protection from myocardial ischemia and perfusion.[25]
See also
References
- ^ FSF Editorial Staff (May–June 1997). "Wheel-well Stowaways Risk Lethal Levels of Hypoxia and Hypothermia" (PDF). Human Factors and Aviation Medicine. 44 (3): 2.
- ^ Kandel, E.R. (2001). "The molecular biology of memory storage: A dialogue between genes and synapses". Science. 294 (5544): 1030–1038. Bibcode:2001Sci...294.1030K. CiteSeerX 10.1.1.322.6795. doi:10.1126/science.1067020. PMID 11691980. S2CID 52799866.
- ^ Hayashi, F.; Coles, S.K.; Bach, K.B.; Mitchell, G.S.; McCrimmon, D.R. (1993). "Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats". Am J Physiol Regul Integr Comp Physiol. 265 (4): R811–819. doi:10.1152/ajpregu.1993.265.4.R811. PMID 8238451.
- ^ a b Fuller, D.D.; Bach, K.B.; Baker, T.L.; Kinkead, R.; Mitchell, G.S. (2000). "Long term facilitation of phrenic motor output". Respir Physiol. 121 (2–3): 135–146. doi:10.1016/S0034-5687(00)00124-9. PMID 10963770.
- ^ Millhorn, D.E.; Eldridge, F.L.; Waldrop, T.G. (1980). "Prolonged stimulation of respiration by a new central neural mechanism". Respir Physiol. 41 (1): 87–103. doi:10.1016/0034-5687(80)90025-0. PMID 6771859.
- ^ Fuller, D.D.; Zabka, A.G.; Baker, T.L.; Mitchell, G.S. (2001). "Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia". J Appl Physiol. 90 (5): 2001–2006. doi:10.1152/jappl.2001.90.5.2001. PMID 11299296.
- ^ a b Baker-Herman, T.L.; Fuller, D.D.; Bavis, R.W.; Zabka, A.G.; Golder, F.J.; Doperalski, N.J.; Johnson, R.A.; Watters, J.J.; Mitchell, G.S. (2004). "BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia". Nat Neurosci. 7 (1): 48–55. doi:10.1038/nn1166. PMID 14699417. S2CID 22965093.
- ^ Hoffman, M.S.; Mitchell, G.S. (2011). "Spinal 5-HT7 receptor activation induces long-lastingphrenic motor facilitation". J Physiol. 589 (6): 1397–1407. doi:10.1113/jphysiol.2010.201657. PMC 3082099. PMID 21242254.
- ^ Wilkerson, J.E.; Mitchell, G.S. (2009). "Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation". Exp Neurol. 217 (1): 116–123. doi:10.1016/j.expneurol.2009.01.017. PMC 2691872. PMID 19416672.
- ^ Sibigtroth, C.M.; Mitchell, G.S. (2011). "Carotid chemoafferent activity is not necessary for all phrenic long-term facilitation following acute intermittent hypoxia". Respir Physiol Neurobiol. 176 (3): 73–79. doi:10.1016/j.resp.2010.11.006. PMC 4374991. PMID 21093615.
- ^ Manukhina, D.B.; Downey, H.F.; Mallet, R.T. (2006). "Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia". Exp Biol Med. 231 (4): 343–365. doi:10.1007/0-387-29540-2_6. PMID 16565431.
- ^ a b Lyamina, N.P.; Lyamina, S.V.; Senchiknin, V.N.; Mallet, R.T.; Downey, H.F.; Manukhina, E.B. (2011). "Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension". J Hypertens. 29 (11): 2265–2272. doi:10.1097/HJH.0b013e32834b5846. PMID 21897291. S2CID 28868197.
- ^ a b Guner, I.; Uzun, D.D.; Yaman, M.O.; Genc, H.; Gelisgen, R.; Korkmaz, G.G.; Hallac, M.; Yelman, N.; Sahin, G.; Karter, Y.; Simsek, G. (2013). "The effect of chronic long-term intermittent hypobaric hypoxia on bone mineral density in rats: role of nitric oxide". Biol Trace Elem Res. 154 (2): 262–267. doi:10.1007/s12011-013-9722-8. PMID 23771686. S2CID 14365564.
- ^ Baker, T.L.; Mitchell, G.S. (2000). "Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats". J Physiol. 529 (1): 215–219. doi:10.1111/j.1469-7793.2000.00215.x. PMC 2270180. PMID 11080263.
- ^ a b Lovett-Barr, M.R.; Satriotomo, I.; Muir, G.D.; Wilkerson, J.E.; Hoffman, M.S.; Vinit, S.; Mitchell, G.S. (2012). "Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury". J Neurosci. 32 (11): 3591–3600. doi:10.1523/JNEUROSCI.2908-11.2012. PMC 3349282. PMID 22423083.
- ^ Gerst, D.G.; 3rd Yokohana, S.S.; Carney, L.M.; Lee, D.S.; Badr, M.S.; Qureshi, T.; Anthouard, M.N.; Mateika, J.H. (2011). "The hypoxic ventilatory response and ventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia". J Appl Physiol. 110 (1): 15–28. doi:10.1152/japplphysiol.00524.2010. PMC 3785116. PMID 20724571.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Haider, T.; Casucci, G.; Linser, T.; Faulhaber, M.; Gatterer, H.; Ott, G.; Linser, A.; Ehrenbourg, I.; Tkatchouk, E.; Burtscher, M.; Bernardi, L. (2009). "Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease". J Hypertens. 27 (8): 1648–1654. doi:10.1097/HJH.0b013e32832c0018. PMID 19387363. S2CID 11277060.
- ^ Hayes, H.B.; Jayataman, A.; Herrmann, M.; Mitchell, G.S.; Rymer, W.Z.; Trumbower, R.D. (2014). "Daily intermittent hypoxia enhances walking after chronic spinal cord injury: A randomized trial". Neurology. 82 (2): 104–113. doi:10.1212/01.WNL.0000437416.34298.43. PMC 3897437. PMID 24285617.
- ^ Lu, X.J.; Chen, X.Q.; Weng, J.; Zhang, H.Y.; Pak, D.T.; Luo, J.H.; Du, J.Z. (2009). "Hippocampal spine-associated Rap-specific GTPase-activating protein induces enhancement of learning and memory in postnatally hypoxia-exposed mice". Neuroscience. 162 (2): 404–414. doi:10.1016/j.neuroscience.2009.05.011. PMC 3243647. PMID 19442707.
- ^ Zhang, J.X.; Chen, X.Q.; Du, J.Z.; Chen, Q.M.; Zhu, C.Y. (2005). "Neonatal exposure to intermittent hypoxia enhances mice performance in water maze and 8-arm radial maze tasks". J Neurobiol. 65 (1): 72–84. doi:10.1002/neu.20174. PMID 16010673.
- ^ Shao, G.; Zhang, R.; Wang, Z.L.; Gao, C.Y.; Huo, X.; Lu, G.W. (2006). "Hippocampal spine-associated Rap-specific GTPase-activating protein induces enhancement of learning and memory in postnatally hypoxia-exposed mice". Neuro-Signals. 15 (6): 314–321. doi:10.1159/000121368. PMID 18349553.
- ^ Zhu, X.H.; Yan, H.C.; Zhang, J.; Qu, H.D.; Qiu, X.S.; Chen, L.; Li, S.J.; Cao, X.; Bean, J.C.; Chen, L.H.; Qin, X.H.; Liu, J.H.; Bai, X.C.; Mei, L.; Gao, T.M. (2010). "Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats". J Neurosci. 30 (38): 12653–12663. doi:10.1523/JNEUROSCI.6414-09.2010. PMC 6633584. PMID 20861371.
- ^ Shatillo, V.B.; Korkushko, O.V.; Ischuk, V.A.; Downey, H.F.; Serebrovskaya, T.V. (2008). "Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men". High Alt Med Biol. 9 (1): 43–52. doi:10.1089/ham.2008.1053. PMID 18331220.
- ^ Bolli, R. (2001). "Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research". J Mol Cell Cardiol. 33 (11): 1897–1918. doi:10.1006/jmcc.2001.1462. PMID 11708836.
