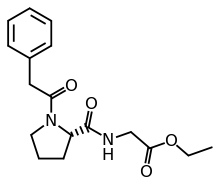
N-Phenylacetyl-L-prolylglycine ethyl ester
This article needs more reliable medical references for verification or relies too heavily on primary sources. (January 2021) |  |
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Noopept |
| Other names | omberacetam; GVS-111; DVD-111; SGS-111; benzylcarbonyl-Pro-Gly-OEt |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H22N2O4 |
| Molar mass | 318.373 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
N-Phenylacetyl-l-prolylglycine ethyl ester is promoted as a nootropic and is a prodrug of cyclic glycine-proline.[a][2] Other names include the brand name Noopept (Template:Lang-ru), developmental code GVS-111; proposed INN omberacetam.[2][3][4]
Its synthesis was first reported in 1996.[2] It is orally available, as of 2017 its metabolism and elimination half-life were not well understood, and cycloprolylglycine has not been measured in humans following administration.[2] In cell culture, cycloprolylglycine increases brain derived neurotrophic factor (BDNF).[5]
It has been evaluated for neuroprotective effects in treating brain injuries and stroke.[6]
Pharmacology
One oft-cited study (originally published in Russian) conducted on rats, suggests that Noopept works via the "antioxidant effect, the anti-inflammatory action, and the ability to inhibit the neurotoxicity of excess calcium and glutamate, and to improve the blood rheology".[6]
Some studies suggest that the pharmacological properties of Noopept are derived from its action as an activator of Hypoxia-inducible factor (HIF-1).[7][8]

Most of the effects of Noopept could be explained by its action as an activator of HIF-1.[8]
Dosage
Noopept is frequently dosed at 10-30mg per day. However, there is no solid evidence indicating that any dose of Noopept is optimal. Few human trials have ever been carried out on Noopept, and as one meta-analysis notes, animal studies have used doses ranging from 0.1mg/kg bodyweight to 10mg/kg bodyweight.[unreliable medical source?][9] Furthermore, no long-term studies have been done to evaluate the lasting effects of chronic use at any given dose; the longest human study lasted for 56 days.[10] There is, therefore, no dose of Noopept which may be called "safe".
Legal status
- Hungary: As of 25 August 2020, Noopept is added to the controlled psychoactive substances list, prohibiting production, sale, import, storage and use.[11]
- Russia: Noopept in Russia is a drug of medicine and is available without a prescription.[12]
- United Kingdom: Contrary to popular belief, omberacetam is not illegal to produce, supply, or import under the Psychoactive Substance Act in the UK, which came into effect on May 26, 2016 because it does neither work as a CNS (central nervous system) depressant, nor as a CNS stimulant.[13] However, sale and supply for human consumption are prohibited.
- United States: The Food and Drug Administration has issued import alerts for imports of omberacetam, considering it an analog of piracetam.[14] FDA considers such racetam-family substances Active Pharmaceutical Ingredients (APIs) that require new drug applications and adequate labelling before being imported.[15] Similarly, warnings have been issued for claims of medical and pharmacological effects.[16] Despite these FDA enforcement actions, omberacetam is sold in over-the-counter supplements in this US with some products formulated with dosages greater than pharmaceutical levels.[17]
See also
Notes
- ^ Referring to the cyclic dipeptide better known as cyclo(prolylglycyl), i.e. (S)-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione.[1] Not to be confused with a cyclopropanyl moiety.
References
- ^ "Omberacetam". Inxight. National Center for Advancing Translational Sciences (NCATS). 4QBJ98683M.
- ^ a b c d "Noopept Information". Examine.com. Retrieved 6 April 2017.
- ^ "Proposed INN List 117". WHO Drug Information. 31 (2): 308. 2017.
- ^ "Omberacetam". AdisInsight. Springer Nature Switzerland AG. Retrieved 12 May 2018.
Alternative Names: DVD-111; GVS 111; Noopept
- ^ Gudasheva TA, Koliasnikova KN, Antipova TA, Seredenin SB (July 2016). "Neuropeptide cycloprolylglycine increases the levels of brain-derived neurotrophic factor in neuronal cells". Doklady. Biochemistry and Biophysics. 469 (1): 273–276. doi:10.1134/S1607672916040104. PMID 27599510. S2CID 254426990.
- ^ a b Ostrovskaia RU, Gudasheva TA, Voronina TA, Seredenin SB (2002). "[The original novel nootropic and neuroprotective agent noopept]" [The original novel nootropic and neuroprotective agent noopept]. Eksperimental'naia i Klinicheskaia Farmakologiia [Experimental and Clinical Pharmacology] (in Russian). 65 (5): 66–72. PMID 12596521.
- ^ Zainullina LF, Ivanova TV, Sadovnikov SV, Vakhitova YV, Seredenin SB (September 2020). "Cognitive Enhancer Noopept Activates Transcription Factor HIF-1". Doklady. Biochemistry and Biophysics. 494 (1): 256–260. doi:10.1134/S1607672920050129. PMID 33119829. S2CID 226207175.
- ^ a b Vakhitova YV, Sadovnikov SV, Borisevich SS, Ostrovskaya RU, A Gudasheva T, Seredenin SB (2016). "Molecular Mechanism Underlying the Action of Substituted Pro-Gly Dipeptide Noopept". Acta Naturae. 8 (1): 82–89. doi:10.32607/20758251-2016-8-1-82-89. PMC 4837574. PMID 27099787.
- ^ Tardner P (2020). "Finding the optimal dosage fornootropic agent Noopept: An analysis of available literature" (PDF). International Journal of Environmental Science and Technology.
- ^ Neznamov GG, Teleshova ES (March 2009). "Comparative studies of Noopept and piracetam in the treatment of patients with mild cognitive disorders in organic brain diseases of vascular and traumatic origin". Neuroscience and Behavioral Physiology. 39 (3): 311–321. doi:10.1007/s11055-009-9128-4. PMID 19234797. S2CID 3348153.
- ^ Miklós K (25 August 2020). "Az új pszichoaktív anyaggá minősített anyagokról vagy vegyületcsoportokról szóló 55/2014. (XII. 30.) EMMI rendelet módosításáról" [About substances classified as new psychoactive substances or 55/2014 on groups of compounds (XII. 30.) amending the EMMI Decree]. Magyarország Hivatalos Lapja [Official Journal of Hungry] (in Hungarian). 194: 6135–6142 (6139). Retrieved 28 April 2021.
- ^ "Ноопепт" [Noopept]. Государственный реестр лекарственных средств [State Register of Medicines] (in Russian).
- ^ "Psychoactive Substances Act 2016". Legislation.gov.uk.
- ^ Cohen PA, Zakharevich I, Gerona R (March 2020). "Presence of Piracetam in Cognitive Enhancement Dietary Supplements". JAMA Internal Medicine. 180 (3): 458–459. doi:10.1001/jamainternmed.2019.5507. PMC 6902196. PMID 31764936.
- ^ "Import alert 66-66". U.S. Food and Drug Administration. 7 September 2021.
- ^ Correll Jr WA (5 February 2019). "Peak Nootropics LLC aka Advanced Nootropics". FDA Warning letter. U.S. Food and Drug Administration.
- ^ Cohen PA, Avula B, Wang YH, Zakharevich I, Khan I (June 2021). "Five Unapproved Drugs Found in Cognitive Enhancement Supplements". Neurology. Clinical Practice. 11 (3): e303–e307. doi:10.1212/CPJ.0000000000000960. PMC 8382366. PMID 34484905.
