Dialkylbiaryl phosphine ligands
Dialkylbiaryl phosphine ligands are phosphine ligands that are used in homogeneous catalysis. They have proved useful in Buchwald-Hartwig amination and etherification reactions as well as Negishi cross-coupling, Suzuki-Miyaura cross-coupling, and related reactions.[1] In addition to these Pd-based processes, their use has also been extended to transformations catalyzed by nickel,[2] gold,[3][4][5] silver,[6] copper,[7] rhodium,[8][9] and ruthenium,[10][11] among other transition metals.[12]
History
Dialkylbiaryl phosphine ligands were first described by Stephen L. Buchwald in 1998 for applications in palladium-catalyzed coupling reactions to form carbon-nitrogen and carbon-carbon bonds.[13] Before their development, use of first- or second-generation phosphine ligands for Pd-catalyzed C-N bond-forming cross-coupling (e.g., tris(o-tolyl)phosphine and BINAP, respectively) necessitated harsh conditions, and the scope of the transformation was severely limited. The Suzuki-Miyaura and Negishi cross-coupling reactions were typically performed with Pd(PPh3)4 as catalyst and were mostly limited to aryl bromides and iodides at elevated temperatures, while the widely available aryl chlorides were unreactive. Dialkylbiaryl phosphine ligands are sometimes referred to as the "Buchwald ligands."[14]
General features
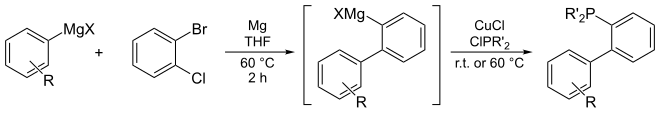
Dialkylbiaryl phosphine ligands are air-stable solids. Many are available commercially. They often can be synthesized in from inexpensive starting materials. One pot protocols have been conducted on >10 kg scales.[15][16]

Their enhanced catalytic activity over other ligands in palladium-catalyzed coupling reactions have been attributed to their electron-richness, steric bulk, and some special structural features. In particular, cyclohexyl, t-butyl, and adamantyl groups on the phosphorus are used for this purpose as bulky, electron-donating substituents. The lower ring of the biphenyl system, ortho to the phosphino group, is also a key structural feature. Numerous crystallographic studies have indicated that it behaves as a hemilabile ligand and is believed to play a role in stabilizing the highly reactive, formally 12-electron L–Pd0 intermediate during the catalytic cycle. 2,6-Substitution on the lower ring minimizes catalyst decomposition via Pd-mediated C-H activation of these positions. Extensive experimentation by the Buchwald group has shown that further minor changes to the structure of these ligands can dramatically alter their catalytic activity in cross coupling reactions with different substrates. This has led to the evolution of multiple ligands that are tailored for specific transformations.[17] By providing a means of generating the postulated catalytically active L–Pd0 species under mild conditions (room temperature or lower in many cases), the development of several generations of base-activated, cyclopalladated precatalysts have further broadened the applicability of the ligands and simplified their use.[18][19]
Common Dialkylbiaryl phosphine ligands
DavePhos
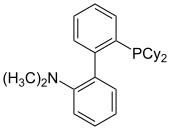
DavePhos, the first reported dialkylbiaryl phosphine ligand, was initially used in Pd-catalyzed Suzuki-Miyaura cross-coupling reactions as well as Buchwald-Hartwig aminations.[20] Complexes of this ligand also catalyze a wide array of reactions, including the arylation of ketones[21] and esters,[22] borylation of aryl chlorides,[23] and the arylation of indoles.[24]
Many modified versions of DavePhos have been synthesized. t-BuDavePhos has been shown to be an even more reactive variant of DavePhos in the room temperature Suzuki-Miyaura coupling of aryl bromides and chlorides.[25] The biphenyl equivalent (PhDavePhos) is also available.
JohnPhos
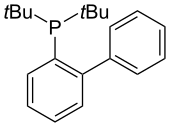
JohnPhos supports the Pd-catalyzed Suzuki-Miyaura reactions with aryl bromides and chlorides.[26] It tolerates hindered substrates and operates at room temperature with low catalyst loading. This ligand has been utilized in multiple reactions including the amination of a range of aryl halides and triflates[27][28] as well as the arylation of thiophenes.[29]
MePhos

Like DavePhos and JohnPhos, MePhos is competent in the Pd-catalyzed Suzuki-Miyaura coupling.[30] It can also form the active catalyst in the formation of aryl ketones.[31] Variants of this ligand, including t-BuMePhos, are also commercially available.
The Pd2(dba)3/MePhos catalytic system has been applied to late stage Suzuki cross couplings. This reaction has been conducted on a kilogram scale, and no specific palladium-removal treatment was required as the excess imidazole present in the final amide coupling step coordinated to the Pd and generated a removable byproduct.[32]

XPhos
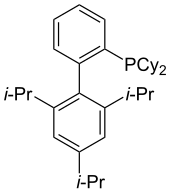
XPhos supports Pd-based catalysts for amination and amidation of arylsulfonates and aryl halides.[33] XPhos has also been used in the Pd-catalyzed borylation of aryl and heteroaryl chlorides[34]
Modified versions of XPhos, he more hindered t-BuXPhos and Me4tButylXPhos, have been employed in the formation of diaryl ethers.[35] Incorporation of a sulfonate group at the 4-position allows this ligand to be used for Sonogashira couplings in aqueous biphasic solvents.[36]
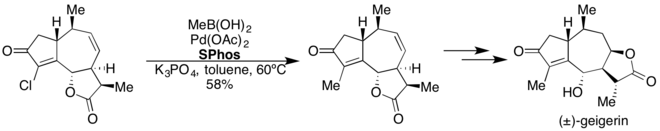
SPhos

SPhos has proven effective in Pd-catalyzed Suzuki-Miyaura coupling reactions.[37] This ligand enables the cross-coupling of heteroaryl, electron-rich and electron-poor aryl, and vinylboronic acids with a variety of aryl and heteroaryl halides under mild reaction conditions. SPhos has also been used in the Pd-catalyzed borylation of aryl and heteroaryl chlorides.[38]
3-Sulfonate variants of sSPhos have been used in Suzuki-Miyaura couplings in aqueous media.[39] SPhos was used in the 8 step total synthesis of (±)-geigerin.[40]
RuPhos

RuPhos has proven effective for Pd-catalyzed Negishi coupling of organozincs with aryl halides.[41] This ligands tolerates hindered substrates as well as a wide range of functional groups. Its complexes also catalyze the trifluoromethylation of aryl chlorides[42] and aminations of aryl halides.[43]
BrettPhos
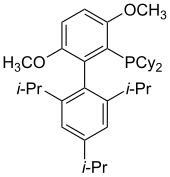
BrettPhos has been evaluated for the Pd-catalyzed amination of aryl mesylates and aryl halides.[44] Pd-BrettPhos complexes catalyze the coupling of weak nucleophiles with aryl halides. Such catalysts are selective for the monoarylation of primary amines. Other applications of BrettPhos in catalysis include trifluoromethylation of aryl chlorides,[45] the formation of aryl trifluoromethyl sulfides,[46] and Suzuki-Miyaura cross-couplings.[47]
Pd- t-BuBrettPhos complexes catalyze the conversion of aryl triflates and aryl bromides to aryl fluorides[48] as well as the synthesis of aromatic nitro compounds.[49] The bulky AdBrettPhos can be used in the amidation of five-membered heterocyclic halides that contain multiple heteroatoms (such as haloimidazoles and halopyrazoles).[50]
CPhos

CPhos has been used as a ligand in the Pd-catalyzed synthesis of 3-cyclopentylindole derivatives,[51] dihydrobenzofurans,[52] and trans-bicyclic sulfamides.[53] It has also been used to synthesize palladacycle precatalysts for Negishi coupling of secondary alkylzinc reagents with aryl halides.[54][55][56]
AlPhos
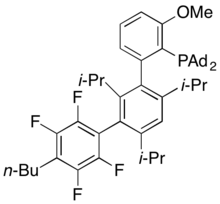
AlPhos allows for the mild Pd-catalyzed fluorination of aryl- and heteroaryl triflates.[57] Reported in 2015, this ligand has been used for Buchwald-Hartwig cross-coupling reactions and synthesizing highly regioselective aryl fluorides through Pd-catalyzed fluorination of various activated aryl and heteroaryl triflates and bromides.[58][59] Its palladium complexes have also been used to prepare aryl thioethers by C–S cross-coupling of thiols with aromatic electrophiles.[60]
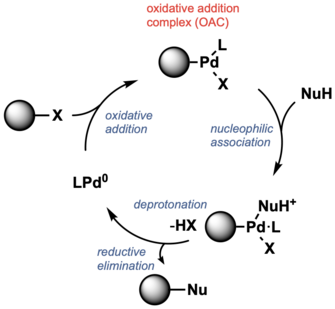
Oxidative addition complex
Many Pd-catalyzed cross coupling reactions involve oxidative addition to form Pd(II) derivatives called oxidative addition complexes (OAC). The resulting L–PdII(Ar)X OAC is electrophilic such that it reacts with a nucleophile and forms C–C and C–heteroatom bonds, after reductive elimination.[61] Such PdIIOACs have been used as precatalysts.[62] OACs exhibit stability, which allows reactions to proceed under mild conditions. They have been applied to bioconjugation.[63] For example, RuPhos and SPhos have been used as ligands for Pd-mediated cysteine arylation, and the use of BrettPhos and t-BuBrettPhos are critical for lysine arylation.[64][65][66][67]
See also
- Coupling reaction
- Organometallic chemistry
- Ligand
- Bioconjugation: Transition Metal-Mediated Bioconjugation Reactions
References
- ^ Surry, David S.; Buchwald, Stephen L. (2008-08-11). "Biaryl Phosphane Ligands in Palladium-Catalyzed Amination". Angewandte Chemie International Edition. 47 (34): 6338–6361. doi:10.1002/anie.200800497. ISSN 1521-3773. PMC 3517088. PMID 18663711.
- ^ Newman-Stonebraker, Samuel H.; Wang, Jason Y.; Jeffrey, Philip D.; Doyle, Abigail G. (October 2022). "Structure–Reactivity Relationships of Buchwald-Type Phosphines in Nickel-Catalyzed Cross-Couplings" (PDF). Journal of the American Chemical Society. 144 (42): 19635–19648. doi:10.1021/jacs.2c09840. PMID 36250758. S2CID 252917338.
- ^ Ferrer, Catalina; Amijs, Catelijne H. M.; Echavarren, Antonio M. (2007-02-02). "Intra- and Intermolecular Reactions of Indoles with Alkynes Catalyzed by Gold". Chemistry - A European Journal. 13 (5): 1358–1373. doi:10.1002/chem.200601324. PMID 17206736.
- ^ Ferrer, Catalina; Echavarren, Antonio M. (2006-02-06). "Gold-Catalyzed Intramolecular Reaction of Indoles with Alkynes: Facile Formation of Eight-Membered Rings and an Unexpected Allenylation". Angewandte Chemie (in German). 118 (7): 1123–1127. doi:10.1002/ange.200503484. ISSN 0044-8249.
- ^ López, Salomé; Herrero-Gómez, Elena; Pérez-Galán, Patricia; Nieto-Oberhuber, Cristina; Echavarren, Antonio M. (2006-09-11). "Gold(I)-Catalyzed Intermolecular Cyclopropanation of Enynes with Alkenes: Trapping of Two Different Gold Carbenes". Angewandte Chemie (in German). 118 (36): 6175–6178. doi:10.1002/ange.200602448. ISSN 0044-8249.
- ^ Porcel, Susana; Echavarren, Antonio M. (2007-03-06). "Intramolecular Carbostannylation of Alkynes Catalyzed by Silver(I) Species". Angewandte Chemie. 119 (15): 2726–2730. doi:10.1002/ange.200605041.
- ^ Haider, Joachim; Kunz, Klaus; Scholz, Ulrich (June 2004). "Highly Selective Copper-Catalyzed Monoarylation of Aniline". Advanced Synthesis & Catalysis. 346 (7): 717–722. doi:10.1002/adsc.200404011. ISSN 1615-4150.
- ^ Dhondi, Pawan K.; Chisholm, John D. (2006-01-01). "Rhodium-Catalyzed Addition of Alkynes to Activated Ketones and Aldehydes". Organic Letters. 8 (1): 67–69. doi:10.1021/ol0525260. ISSN 1523-7060. PMID 16381569.
- ^ Dhondi, Pawan K.; Carberry, Patrick; Choi, Lydia B.; Chisholm, John D. (2007-12-01). "Addition of Alkynes to Aldehydes and Activated Ketones Catalyzed by Rhodium−Phosphine Complexes". The Journal of Organic Chemistry. 72 (25): 9590–9596. doi:10.1021/jo701643h. ISSN 0022-3263. PMID 17999525.
- ^ Movassaghi, Mohammad; Hill, Matthew D. (2006-11-01). "Single-Step Synthesis of Pyrimidine Derivatives". Journal of the American Chemical Society. 128 (44): 14254–14255. doi:10.1021/ja066405m. ISSN 0002-7863. PMID 17076488.
- ^ Faller, J. W.; D'Alliessi, Darlene G. (2003-06-01). "Planar Chirality in Tethered η 6 :η 1 -(Phosphinophenylenearene -P )ruthenium(II) Complexes and Their Potential Use as Asymmetric Catalysts". Organometallics. 22 (13): 2749–2757. doi:10.1021/om030080q. ISSN 0276-7333.
- ^ Surry, David S.; Buchwald, Stephen L. (2011). "Dialkylbiaryl phosphines in Pd-catalyzed amination: a user's guide". Chem. Sci. 2 (1): 27–50. doi:10.1039/C0SC00331J. ISSN 2041-6539. PMC 3306613. PMID 22432049.
- ^ Old, David W.; Wolfe, John P.; Buchwald, Stephen L. (September 1998). "A Highly Active Catalyst for Palladium-Catalyzed Cross-Coupling Reactions: Room-Temperature Suzuki Couplings and Amination of Unactivated Aryl Chlorides". Journal of the American Chemical Society. 120 (37): 9722–9723. doi:10.1021/ja982250+.
- ^ "Buchwald Phosphine Ligands". Sigma-Aldrich. Retrieved 2023-06-08.
- ^ Martin, Ruben; Buchwald, Stephen L. (18 November 2008). "Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands". Accounts of Chemical Research. 41 (11): 1461–1473. doi:10.1021/ar800036s. ISSN 0001-4842. PMC 2645945. PMID 18620434.
- ^ Kaye, Steven; Fox, Joseph M.; Hicks, Frederick A.; Buchwald, Stephen L. (31 December 2001). "The Use of Catalytic Amounts of CuCl and Other Improvements in the Benzyne Route to Biphenyl-Based Phosphine Ligands". Advanced Synthesis & Catalysis. 343 (8): 789–794. doi:10.1002/1615-4169(20011231)343:8<789::AID-ADSC789>3.0.CO;2-A. ISSN 1615-4169.
- ^ Martin, Ruben; Buchwald, Stephen L. (18 November 2008). "Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands". Accounts of Chemical Research. 41 (11): 1461–1473. doi:10.1021/ar800036s. ISSN 0001-4842. PMC 2645945. PMID 18620434.
- ^ Biscoe, Mark R.; Fors, Brett P.; Buchwald, Stephen L. (2008-05-01). "A New Class of Easily Activated Palladium Precatalysts for Facile C−N Cross-Coupling Reactions and the Low Temperature Oxidative Addition of Aryl Chlorides". Journal of the American Chemical Society. 130 (21): 6686–6687. doi:10.1021/ja801137k. PMC 2587037. PMID 18447360.
- ^ Bruno, N. C.; Buchwald, S. L. (2014). Palladium Precatalysts for Cross-Coupling Reactions (PDF). The Strem Chemiker.
- ^ Old, David W.; Wolfe, John P.; Buchwald, Stephen L. (September 1998). "A Highly Active Catalyst for Palladium-Catalyzed Cross-Coupling Reactions: Room-Temperature Suzuki Couplings and Amination of Unactivated Aryl Chlorides". Journal of the American Chemical Society. 120 (37): 9722–9723. doi:10.1021/ja982250+.
- ^ Fox, Joseph M.; Huang, Xiaohua; Chieffi, André; Buchwald, Stephen L. (1 February 2000). "Highly Active and Selective Catalysts for the Formation of α-Aryl Ketones". Journal of the American Chemical Society. 122 (7): 1360–1370. doi:10.1021/ja993912d. ISSN 0002-7863.
- ^ Moradi, Wahed A.; Buchwald, Stephen L. (2001). "Palladium-Catalyzedα-Arylation of Esters". Journal of the American Chemical Society. 123 (33): 7996–8002. doi:10.1021/ja010797+. ISSN 0002-7863. PMID 11506555.
- ^ Billingsley, Kelvin L.; Barder, Timothy E.; Buchwald, Stephen L. (9 July 2007). "Palladium-Catalyzed Borylation of Aryl Chlorides: Scope, Applications, and Computational Studies". Angewandte Chemie International Edition. 46 (28): 5359–5363. doi:10.1002/anie.200701551. ISSN 1521-3773. PMID 17562550.
- ^ Old, David W.; Harris, Michele C.; Buchwald, Stephen L. (1 May 2000). "Efficient Palladium-Catalyzed N-Arylation of Indoles". Organic Letters. 2 (10): 1403–1406. doi:10.1021/ol005728z. ISSN 1523-7060. PMID 10814458.
- ^ Wolfe, John P.; Singer, Robert A.; Yang, Bryant H.; Buchwald, Stephen L. (1 October 1999). "Highly Active Palladium Catalysts for Suzuki Coupling Reactions". Journal of the American Chemical Society. 121 (41): 9550–9561. doi:10.1021/ja992130h. ISSN 0002-7863.
- ^ Wolfe, John P.; Singer, Robert A.; Yang, Bryant H.; Buchwald, Stephen L. (1 October 1999). "Highly Active Palladium Catalysts for Suzuki Coupling Reactions". Journal of the American Chemical Society. 121 (41): 9550–9561. doi:10.1021/ja992130h. ISSN 0002-7863.
- ^ Wolfe, John P.; Tomori, Hiroshi; Sadighi, Joseph P.; Yin, Jingjun; Buchwald, Stephen L. (1 February 2000). "Simple, Efficient Catalyst System for the Palladium-Catalyzed Amination of Aryl Chlorides, Bromides, and Triflates" (PDF). The Journal of Organic Chemistry. 65 (4): 1158–1174. doi:10.1021/jo991699y. ISSN 0022-3263. PMID 10814067.
- ^ Surry, David S.; Buchwald, Stephen L. (11 August 2008). "Biaryl Phosphane Ligands in Palladium-Catalyzed Amination". Angewandte Chemie International Edition. 47 (34): 6338–6361. doi:10.1002/anie.200800497. ISSN 1521-3773. PMC 3517088. PMID 18663711.
- ^ Okazawa, Toru; Satoh, Tetsuya; Miura, Masahiro; Nomura, Masakatsu (1 May 2002). "Palladium-Catalyzed Multiple Arylation of Thiophenes". Journal of the American Chemical Society. 124 (19): 5286–5287. doi:10.1021/ja0259279. ISSN 0002-7863. PMID 11996567.
- ^ Wolfe, John P.; Singer, Robert A.; Yang, Bryant H.; Buchwald, Stephen L. (1 October 1999). "Highly Active Palladium Catalysts for Suzuki Coupling Reactions". Journal of the American Chemical Society. 121 (41): 9550–9561. doi:10.1021/ja992130h. ISSN 0002-7863.
- ^ Fox, Joseph M.; Huang, Xiaohua; Chieffi, André; Buchwald, Stephen L. (1 February 2000). "Highly Active and Selective Catalysts for the Formation of α-Aryl Ketones". Journal of the American Chemical Society. 122 (7): 1360–1370. doi:10.1021/ja993912d. ISSN 0002-7863.
- ^ Thiel, Oliver; Achmatowicz, Michal; Milburn, Robert (11 June 2012). "Process Research and Development for Heterocyclic p38 MAP Kinase Inhibitors". Synlett. 23 (11): 1564–1574. doi:10.1055/s-0031-1290425. S2CID 196773656.
- ^ Huang, Xiaohua; Anderson, Kevin W.; Zim, Danilo; Jiang, Lei; Klapars, Artis; Buchwald, Stephen L. (1 June 2003). "Expanding Pd-Catalyzed C−N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions". Journal of the American Chemical Society. 125 (22): 6653–6655. doi:10.1021/ja035483w. ISSN 0002-7863. PMID 12769573.
- ^ Billingsley, Kelvin L.; Barder, Timothy E.; Buchwald, Stephen L. (9 July 2007). "Palladium-Catalyzed Borylation of Aryl Chlorides: Scope, Applications, and Computational Studies". Angewandte Chemie International Edition. 46 (28): 5359–5363. doi:10.1002/anie.200701551. ISSN 1521-3773. PMID 17562550.
- ^ Burgos, Carlos H.; Barder, Timothy E.; Huang, Xiaohua; Buchwald, Stephen L. (26 June 2006). "Significantly Improved Method for the Pd-Catalyzed Coupling of Phenols with Aryl Halides: Understanding Ligand Effects". Angewandte Chemie International Edition. 45 (26): 4321–4326. doi:10.1002/anie.200601253. ISSN 1521-3773. PMID 16733839.
- ^ Anderson, Kevin W.; Buchwald, Stephen L. (26 September 2005). "General Catalysts for the Suzuki–Miyaura and Sonogashira Coupling Reactions of Aryl Chlorides and for the Coupling of Challenging Substrate Combinations in Water". Angewandte Chemie International Edition. 44 (38): 6173–6177. doi:10.1002/anie.200502017. ISSN 1521-3773. PMID 16097019.
- ^ Walker, Shawn D.; Barder, Timothy E.; Martinelli, Joseph R.; Buchwald, Stephen L. (26 March 2004). "A Rationally Designed Universal Catalyst for Suzuki–Miyaura Coupling Processes". Angewandte Chemie International Edition. 43 (14): 1871–1876. doi:10.1002/anie.200353615. ISSN 1521-3773. PMID 15054800.
- ^ Billingsley, Kelvin L.; Barder, Timothy E.; Buchwald, Stephen L. (9 July 2007). "Palladium-Catalyzed Borylation of Aryl Chlorides: Scope, Applications, and Computational Studies". Angewandte Chemie International Edition. 46 (28): 5359–5363. doi:10.1002/anie.200701551. ISSN 1521-3773. PMID 17562550.
- ^ Anderson, Kevin W.; Buchwald, Stephen L. (26 September 2005). "General Catalysts for the Suzuki–Miyaura and Sonogashira Coupling Reactions of Aryl Chlorides and for the Coupling of Challenging Substrate Combinations in Water". Angewandte Chemie International Edition. 44 (38): 6173–6177. doi:10.1002/anie.200502017. ISSN 1521-3773. PMID 16097019.
- ^ Carret, Sébastien; Deprés, Jean-Pierre (10 September 2007). "Access to Guaianolides: Highly Efficient Stereocontrolled Total Synthesis of (±)-Geigerin". Angewandte Chemie International Edition. 46 (36): 6870–6873. doi:10.1002/anie.200702031. ISSN 1521-3773. PMID 17676568.
- ^ Milne, Jacqueline E.; Buchwald, Stephen L. (1 October 2004). "An Extremely Active Catalyst for the Negishi Cross-Coupling Reaction". Journal of the American Chemical Society. 126 (40): 13028–13032. doi:10.1021/ja0474493. ISSN 0002-7863. PMID 15469301.
- ^ Cho, Eun Jin; Senecal, Todd D.; Kinzel, Tom; Zhang, Yong; Watson, Donald A.; Buchwald, Stephen L. (25 June 2010). "The Palladium-Catalyzed Trifluoromethylation of Aryl Chlorides". Science. 328 (5986): 1679–1681. Bibcode:2010Sci...328.1679C. doi:10.1126/science.1190524. ISSN 0036-8075. PMC 3005208. PMID 20576888.
- ^ Charles, Mark D.; Schultz, Phillip; Buchwald, Stephen L. (1 September 2005). "Efficient Pd-Catalyzed Amination of Heteroaryl Halides". Organic Letters. 7 (18): 3965–3968. doi:10.1021/ol0514754. ISSN 1523-7060. PMID 16119943.
- ^ Fors, Brett P.; Watson, Donald A.; Biscoe, Mark R.; Buchwald, Stephen L. (15 October 2008). "A Highly Active Catalyst for Pd-Catalyzed Amination Reactions: Cross-Coupling Reactions Using Aryl Mesylates and the Highly Selective Monoarylation of Primary Amines Using Aryl Chlorides". Journal of the American Chemical Society. 130 (41): 13552–13554. doi:10.1021/ja8055358. ISSN 0002-7863. PMC 2748321. PMID 18798626.
- ^ Cho, Eun Jin; Senecal, Todd D.; Kinzel, Tom; Zhang, Yong; Watson, Donald A.; Buchwald, Stephen L. (25 June 2010). "The Palladium-Catalyzed Trifluoromethylation of Aryl Chlorides". Science. 328 (5986): 1679–1681. Bibcode:2010Sci...328.1679C. doi:10.1126/science.1190524. ISSN 0036-8075. PMC 3005208. PMID 20576888.
- ^ Teverovskiy, Georgiy; Surry, David S.; Buchwald, Stephen L. (1 August 2011). "Pd-Catalyzed Synthesis of Ar-SCF3 Compounds under Mild Conditions". Angewandte Chemie International Edition. 50 (32): 7312–7314. doi:10.1002/anie.201102543. ISSN 1521-3773. PMC 3395331. PMID 21692157.
- ^ Bhayana, Brijesh; Fors, Brett P.; Buchwald, Stephen L. (3 September 2009). "A Versatile Catalyst System for Suzuki−Miyaura Cross-Coupling Reactions of C(sp2)-Tosylates and Mesylates". Organic Letters. 11 (17): 3954–3957. doi:10.1021/ol9015892. ISSN 1523-7060. PMC 2759755. PMID 19663467.
- ^ Watson, Donald A.; Su, Mingjuan; Teverovskiy, Georgiy; Zhang, Yong; García-Fortanet, Jorge; Kinzel, Tom; Buchwald, Stephen L. (25 September 2009). "Formation of ArF from LPdAr(F): Catalytic Conversion of Aryl Triflates to Aryl Fluorides". Science. 325 (5948): 1661–1664. Bibcode:2009Sci...325.1661W. doi:10.1126/science.1178239. ISSN 0036-8075. PMC 3038120. PMID 19679769.
- ^ Fors, Brett P.; Buchwald, Stephen L. (16 September 2009). "Pd-Catalyzed Conversion of Aryl Chlorides, Triflates, and Nonaflates to Nitroaromatics". Journal of the American Chemical Society. 131 (36): 12898–12899. doi:10.1021/ja905768k. ISSN 0002-7863. PMC 2773681. PMID 19737014.
- ^ Su, Mingjuan; Buchwald, Stephen L. (7 May 2012). "A Bulky Biaryl Phosphine Ligand Allows for Palladium-Catalyzed Amidation of Five-Membered Heterocycles as Electrophiles". Angewandte Chemie International Edition. 51 (19): 4710–4713. doi:10.1002/anie.201201244. ISSN 1521-3773. PMC 3407381. PMID 22473747.
- ^ Kirsch, Janelle K.; Manske, Jenna L.; Wolfe, John P. (2018-11-02). "Pd-Catalyzed Alkene Carboheteroarylation Reactions for the Synthesis of 3-Cyclopentylindole Derivatives". The Journal of Organic Chemistry. 83 (21): 13568–13573. doi:10.1021/acs.joc.8b02165. ISSN 0022-3263. PMC 6375689. PMID 30351050.
- ^ Hutt, Johnathon T.; Wolfe, John P. (2016-09-20). "Synthesis of 2,3-dihydrobenzofurans via the palladium catalyzed carboalkoxylation of 2-allylphenols". Organic Chemistry Frontiers. 3 (10): 1314–1318. doi:10.1039/C6QO00215C. ISSN 2052-4129. PMC 5382964. PMID 28392926.
- ^ Babij, Nicholas R.; McKenna, Grace M.; Fornwald, Ryan M.; Wolfe, John P. (2014-06-20). "Stereocontrolled Synthesis of Bicyclic Sulfamides via Pd-Catalyzed Alkene Carboamination Reactions. Control of 1,3-Asymmetric Induction by Manipulating Mechanistic Pathways". Organic Letters. 16 (12): 3412–3415. doi:10.1021/ol5015976. ISSN 1523-7060. PMC 4076003. PMID 24916343.
- ^ Han, Chong; Buchwald, Stephen L. (10 June 2009). "Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides". Journal of the American Chemical Society. 131 (22): 7532–7533. doi:10.1021/ja902046m. ISSN 0002-7863. PMC 2746668. PMID 19441851.
- ^ Yang, Yang; Niedermann, Katrin; Han, Chong; Buchwald, Stephen L. (2014-09-05). "Highly Selective Palladium-Catalyzed Cross-Coupling of Secondary Alkylzinc Reagents with Heteroaryl Halides". Organic Letters. 16 (17): 4638–4641. doi:10.1021/ol502230p. ISSN 1523-7060. PMC 4156254. PMID 25153332.
- ^ Zhang, Hu; Buchwald, Stephen L. (2017-08-23). "Palladium-Catalyzed Negishi Coupling of α-CF 3 Oxiranyl Zincate: Access to Chiral CF3 -Substituted Benzylic Tertiary Alcohols". Journal of the American Chemical Society. 139 (33): 11590–11594. doi:10.1021/jacs.7b06630. ISSN 0002-7863. PMID 28753004.
- ^ "AlPhos and [(AlPhosPd)2•COD] for Pd-Catalyzed Fluorination". Sigma-Aldrich. Retrieved 2018-08-17.
- ^ Sather, Aaron C.; Lee, Hong Geun; De La Rosa, Valentina Y.; Yang, Yang; Müller, Peter; Buchwald, Stephen L. (2015-10-21). "A Fluorinated Ligand Enables Room-Temperature and Regioselective Pd-Catalyzed Fluorination of Aryl Triflates and Bromides". Journal of the American Chemical Society. 137 (41): 13433–13438. doi:10.1021/jacs.5b09308. ISSN 0002-7863. PMC 4721526. PMID 26413908.
- ^ Sather, Aaron C.; Lee, Hong Geun; De La Rosa, Valentina Y.; Yang, Yang; Müller, Peter; Buchwald, Stephen L. (21 October 2015). "A Fluorinated Ligand Enables Room-Temperature and Regioselective Pd-Catalyzed Fluorination of Aryl Triflates and Bromides". Journal of the American Chemical Society. 137 (41): 13433–13438. doi:10.1021/jacs.5b09308. ISSN 0002-7863. PMC 4721526. PMID 26413908.
- ^ Shaughnessy, Kevin H. (March 2020). "Development of Palladium Precatalysts that Efficiently Generate LPd(0) Active Species". Israel Journal of Chemistry. 60 (3–4): 180–194. doi:10.1002/ijch.201900067. ISSN 0021-2148. S2CID 202882630.
- ^ Johansson Seechurn, Carin C. C.; Kitching, Matthew O.; Colacot, Thomas J.; Snieckus, Victor (2012-05-21). "Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize". Angewandte Chemie International Edition. 51 (21): 5062–5085. doi:10.1002/anie.201107017. PMID 22573393.
- ^ Ingoglia, Bryan T.; Buchwald, Stephen L. (2017-06-02). "Oxidative Addition Complexes as Precatalysts for Cross-Coupling Reactions Requiring Extremely Bulky Biarylphosphine Ligands". Organic Letters. 19 (11): 2853–2856. doi:10.1021/acs.orglett.7b01082. ISSN 1523-7060. PMC 5580394. PMID 28498667.
- ^ Uehling, Mycah R.; King, Ryan P.; Krska, Shane W.; Cernak, Tim; Buchwald, Stephen L. (2019-01-25). "Pharmaceutical diversification via palladium oxidative addition complexes". Science. 363 (6425): 405–408. doi:10.1126/science.aac6153. ISSN 0036-8075. PMID 30679373. S2CID 59248487.
- ^ Vinogradova, Ekaterina V.; Zhang, Chi; Spokoyny, Alexander M.; Pentelute, Bradley L.; Buchwald, Stephen L. (October 2015). "Organometallic palladium reagents for cysteine bioconjugation". Nature. 526 (7575): 687–691. doi:10.1038/nature15739. ISSN 0028-0836. PMC 4809359. PMID 26511579.
- ^ Rojas, Anthony J.; Pentelute, Bradley L.; Buchwald, Stephen L. (2017-08-18). "Water-Soluble Palladium Reagents for Cysteine S -Arylation under Ambient Aqueous Conditions". Organic Letters. 19 (16): 4263–4266. doi:10.1021/acs.orglett.7b01911. ISSN 1523-7060. PMC 5818991. PMID 28777001.
- ^ Jbara, Muhammad; Rodriguez, Jacob; Dhanjee, Heemal H.; Loas, Andrei; Buchwald, Stephen L.; Pentelute, Bradley L. (2021-05-17). "Oligonucleotide Bioconjugation with Bifunctional Palladium Reagents". Angewandte Chemie International Edition. 60 (21): 12109–12115. doi:10.1002/anie.202103180. ISSN 1433-7851. PMC 8143041. PMID 33730425.
- ^ Lee, Hong Geun; Lautrette, Guillaume; Pentelute, Bradley L.; Buchwald, Stephen L. (2017-03-13). "Palladium-Mediated Arylation of Lysine in Unprotected Peptides". Angewandte Chemie International Edition. 56 (12): 3177–3181. doi:10.1002/anie.201611202. hdl:1721.1/115190. PMC 5741856. PMID 28206688.