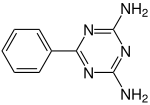
Benzoguanamine
Appearance
| |
| Names | |
|---|---|
| Systematic IUPAC name
6-Phenyl-1,3,5-triazine-2,4-diamine | |
| Other names
Diamino-6-phenyl-1,3,5-triazine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.905 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H9N5 | |
| Molar mass | 187.206 g·mol−1 |
| Appearance | White solid |
| Density | 1.42 g cm−3 |
| Melting point | 227–228 °C (441–442 °F; 500–501 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H302, H331, H332, H412 | |
| P261, P264, P270, P271, P273, P301+P312, P304+P312, P304+P340, P311, P312, P321, P330, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzoguanamine is an organic compound with the chemical formula (CNH2)2(CC6H5)N3. It is related to melamine but with one amino group replaced by phenyl. Benzooguanamine is used in the manufacturing of melamine resins. Unlike melamine ((CNH2)3N3), benzoguanamine is not a crosslinker. The "benzo" prefix is historical, the compound contains phenyl, not a benzo group. A related compound is acetoguanamine.[1]
The compound is prepared by condensation of cyanoguanidine with benzonitrile.[2]
- (H2N)2C=NCN + PhCN → (CNH2)2(CPh)N3
Safety
LD50 (oral, rats) is 1470 mg/kg.
References
- ^ "Amino Resins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2012. doi:10.1002/14356007.a02_115.pub2.
{{cite encyclopedia}}: Unknown parameter|authors=ignored (help) - ^ "Benzoguanamine". Org. Synth. 33: 13. 1953. doi:10.15227/orgsyn.033.0013.
{{cite journal}}: Unknown parameter|authors=ignored (help)
