Benzyl iodide
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Benzyl iodide
| |
| Other names
Fraissite, iodotoluol, α-iodotoluene, phenylmethyliodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.009.659 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H7I | |
| Molar mass | 218.037 g·mol−1 |
| Appearance | Low-melting crystals or colorless liquid |
| Melting point | 24.5 °C |
| Boiling point | 218 °C (424 °F; 491 K) |
| Insoluble | |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| Flash point | 86 °C (187 °F; 359 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
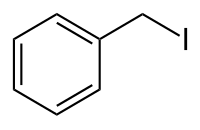
Benzyl iodide is an organic compound with the chemical formula C
7H
7I.[1][2] The compound consists of a benzene ring with an attached iodidemethyl group. The substance is an alkyl halide and is a constitutional isomer of the iodotoluenes.
Synthesis
Benzyl iodide can be obtained via the Finkelstein reaction from benzyl chloride and sodium iodide in acetone.

Properties
Benzyl iodide forms colorless to yellow needles, melting at 24.5 °C.[3] As a liquid, the compound has the high refractive index of 1.6334. Benzyl iodide is also a powerful lachrymator.[4][5]
See also
References
- ^ "BENZYL IODIDE". chemicalbook.com. Retrieved 8 June 2017.
- ^ "Benzyl iodide". NIST. webbook.nist.gov. Retrieved 8 June 2017.
- ^ CRC Handbook of Chemistry and Physics, 90. Edition, CRC Press, Boca Raton, Florida, 2009, ISBN 978-1-4200-9084-0, Section 3, Physical Constants of Organic Compounds, p. 3-306.
- ^ Bauta, William E. (2001). "Benzyl Iodide". Encyclopedia of Reagents for Organic Synthesis. onlinelibrary.wiley.com. doi:10.1002/047084289X.rb060. ISBN 978-0471936237.
- ^ Fieser, Louis F.; Fieser, Mary (1982). Organische Chemie (in German). ISBN 978-3-527-25075-2.
